Chemotherapy for Treatment of Grade II Gliomas
In this article, we provide a brief overview of the management of grade II astrocytomas, oligodendrogliomas, and mixed oligoastrocytomas-the three most heavily encountered and studied of the low-grade gliomas.
Figure: Suggested Treatment Algorithm for Grade II Gliomas Based on Recent Emerging Data From RTOG 9802
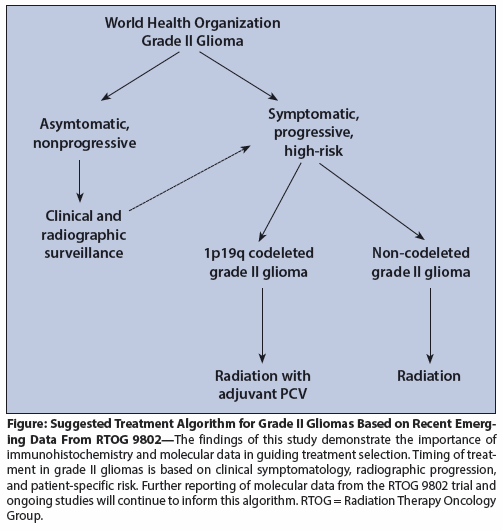
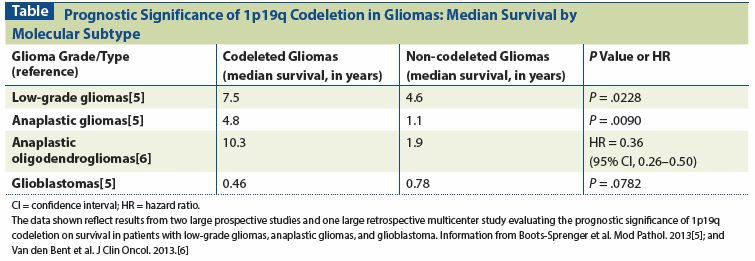
Table: Prognostic Significance of 1p19q Codeletion in Gliomas: Median Survival by Molecular Subtype
Gliomas classified as grade II by the World Health Organization (WHO) include astrocytomas, oligodendrogliomas, and mixed oligoastrocytomas. This heterogeneous group of conditions is associated with a more favorable prognosis and longer-term survival than high-grade gliomas (HGGs). Neurosurgical resection and radiation therapy improve survival in symptomatic, progressive, or high-risk grade II gliomas. Until recently, the role of chemotherapy has been less clear. This review draws on insights from the management of HGGs and emerging data on the addition of PCV (procarbazine, lomustine [CCNU], and vincristine) to radiation for these neoplasms. Specifically, this review focuses on the current status of chemotherapeutic management of grade II gliomas, including optimal timing of treatment, and management of 1p19q codeleted and non-codeleted tumors.
Introduction
Grade II gliomas comprise a heterogeneous group of primary central nervous system (CNS) tumors that vary widely in their histopathology, molecular features, and clinical symptomatology. While diverse, this group of conditions shares a generally indolent course with good prognosis and favorable long-term survival data. The benefits of surgery and radiation therapy (RT) have long been recognized for many types of low-grade lesions in this setting. Emerging data suggest that chemotherapy, although historically reserved primarily for recurrence or progression, may have a substantial impact earlier in the disease course.
In this article, we provide a brief overview of the management of grade II astrocytomas, oligodendrogliomas, and mixed oligoastrocytomas-the three most heavily encountered and studied of the low-grade gliomas (LGGs). For a comprehensive review of other low-grade glial neoplasms, please see recent review articles on this subject.[1]
Classification of Grade II Gliomas: Histologic and Genetic Factors
Grade II gliomas are classified based on the international classification of CNS neoplasms published by the World Health Organization.[2] This classification scheme defines this group based on its histologic features, including hypercellularity, nuclear atypia, pleomorphism, and lack of significant mitotic activity. Lower proliferative indices are common, and necrosis or vascular proliferation, which is diagnostic of high-grade gliomas (HGGs), is not observed. These histopathologic criteria remain the gold standard for diagnosis but are increasingly informed by molecular and immunohistochemical markers.
At present, four genetic markers augment the pathologic evaluation of LGGs and have become increasingly important in understanding and selecting the appropriate treatments for these neoplasms. Combined loss of heterozygosity on the short arm of chromosome 1 and the long arm of chromosome 19 (ie, complete 1p19q codeletion) has become one of the most important markers in the evaluation and management of LGGs. Codeletion of 1p19q reliably differentiates between the more favorable pure oligodendrogliomas and less favorable mixed oligoastrocytomas or astrocytomas. Codeletion is present in at least 75% of all low-grade oligodendrogliomas (LGOs) but only 20% of low-grade astrocytomas (LGAs).[3] Importantly, 1p19q codeletion has been shown repeatedly to have both prognostic and predictive implications. In a recent meta-analysis, codeletion of 1p19q was strongly associated with improved survival in oligodendrogliomas (hazard ratio [HR] = 0.41; 95% confidence interval [CI], 0.30–0.56), in all gliomas (HR = 0.41; 95% CI, 0.34–0.48), and in histologically described astrocytic tumors (HR = 0.52; 95% CI, 0.36–0.75).[4] One large multicenter retrospective study reported an absolute median survival difference of 2.9 years in patients with low-grade gliomas with vs without 1p19q codeletion (P = .0228; see Table).[5] In patients with anaplastic oligodendrogliomas (AOs), the presence of 1p19q codeletion is strongly associated with survival and chemosensitivity to PCV (procarbazine, lomustine [CCNU], and vincristine).[6,7] Thus, this genetic marker has become a key factor in determining the optimal management of oligodendroglial neoplasms and may begin to replace isolated histologic classification.
Other markers have also been shown to support the pathologic evaluation of LGGs but have lesser implications for treatment selection. These include mutations in the isocitrate dehydrogenase 1 and 2 (IDH1/2) genes. Mutation of IDH1 is common and present in over 85% of LGGs.[8] While this marker portends a more favorable prognosis in general, it does not appear to differentiate between LGG subtypes. There is some suggestion that IDH mutational status may also provide implications for treatment selection and response; prospective data are lacking, however, and further study is ongoing. Mutations of the tumor protein 53 (TP53) gene are more common in astrocytic lesions, having been reported in over 50% of LGAs but only 10% of LGOs.[3] Unlike codeletion of 1p19q, expression of TP53 is not predictive of survival or response to treatment. Methylation of the O6-methylguanine-DNA methyltransferase (MGMT) gene promoter has become an increasingly important marker in HGGs, in which it appears to be of prognostic importance. Its prevalence in LGGs is higher, and a prospective study of its predictive value in these lesions is lacking.[9]
Overview of the Management of Grade II Gliomas
Medical treatment of grade II gliomas involves a combination of surgery, RT, and chemotherapy and is best conducted by a multidisciplinary team. Surgery is the backbone of management of these neoplasms, providing de facto tissue diagnosis and gross cytoreduction of tumor. The prognostic importance of the extent of surgical resection has been discussed repeatedly in the literature. Because of ethical concerns, no randomized prospective studies have been performed in glioma patients randomized to different extents of resection. Several large retrospective analyses have consistently demonstrated the extent of resection to be independently associated with freedom from seizures[10] and improved survival, with gross total resection (GTR) found to be more favorable than both subtotal resection (STR) and biopsy.[11-15] Without prospective study, however, it is difficult to determine whether this is a result of the surgery itself or whether surgery is a surrogate for differences in underlying tumor biology. Despite advances in intraoperative magnetic resonance imaging (MRI) and other techniques for improving the extent of resection, due to the infiltrative nature of these lesions, residual tumor remains almost universally, and RT and chemotherapy provide important adjuvant treatments.[16]
RT has been shown repeatedly to improve survival in patients with LGG and has long played an important role in their management.[12,17] A number of chemotherapeutics have been investigated in this setting, including dactinomycin, vincristine, carboplatin, etoposide, PCV, temozolomide (TMZ), and combinations of these agents. In general, these have been studied in small phase II, single-arm trials, which have often evaluated radiographic response. The small number of patients studied, favorable long-term survival associated with LGG, heterogeneous populations with a lack of optimal comparators, and reliance on radiographic response in non–contrast-enhancing tumors has limited the ability to arrive at practice-changing conclusions.
Chemotherapeutic Management of LGG: Emerging Data
In 1998, based on increasing interest in the role of PCV in the management of low- and high-grade gliomas, the Radiation Therapy Oncology Group (RTOG) began a large phase III study (RTOG 9802) that randomized 251 patients with high-risk LGGs to RT (at 54 Gy) with or without adjuvant PCV. Patients with grade II gliomas who were either < 40 years of age with residual radiographic disease or > 40 years of age following any surgical intervention were randomized to RT alone vs RT followed by 6 cycles of PCV. Initial results were published in 2012 and demonstrated improvement in progression-free survival (PFS) but not overall survival (OS).[18] Long-term follow up data became available in early 2014 and were reported at the American Society of Clinical Oncology (ASCO) Annual Meeting.[19] With a median follow-up of 11.9 years and with 55% of patients deceased, the authors reported significantly longer OS among the PCV-treated patients. Patients who were also treated with PCV lived 5.5 years longer than those who were treated with RT alone (13.3 years vs 7.8 years; HR = 0.59; P < .03). In fact, 20% more patients in the combined-treatment arm were alive at 10 years. Improvement in PFS was sustained with combination treatment (10.4 years with PCV plus RT vs 4.0 years with RT alone), with approximately 30% more patients being progression-free at 10 years in the PCV-treated arm (HR = 0.50; P < .002). This difference in OS was observed despite the fact that at progression, more patients in the RT-alone arm received salvage chemotherapy (56% vs 23% in the PCV arm). Furthermore, while on-treatment toxicity was greater in the PCV arm, this was manageable, and no significant differences were observed in long-term cognition as measured by the Mini-Mental State Examination (MMSE), nor have there been reports of late-onset myelodysplasia or leukemia.
A variety of prognostic factors, confounders, and covariates were included in RTOG 9802; however, detailed subgroup analysis by molecular, immunohistochemical, and genetic markers is not yet available. To date, treatment type (ie, inclusion of PCV) and histology were reported to be independently associated with prognosis. Female gender was associated with better OS in the multivariate model, and it should be noted that while baseline characteristics were mostly similar in the two arms, there was a larger percentage of females in the PCV-treated arm (48% vs 39% in the RT-alone arm). Interestingly, extent of surgery was not identified as an independent predictor of PFS or OS despite prior reports to the contrary. Subgroup analysis investigating the association between extent of surgery and molecular phenotype (ie, codeleted vs non-codeleted) is underway and will be important. This dramatic improvement in OS has sparked practice-changing conversations and raised a number of important questions regarding the chemotherapeutic management of grade II gliomas, including: (1) the optimal timing of such treatment, (2) appropriate patient selection and incorporation of genetic data (ie, 1p19q codeletion) into treatment selection, and (3) the appropriateness of substituting other CNS-penetrating alkylating regimens such as TMZ.
Optimal Timing of Treatment in Grade II Gliomas: When to Initiate Therapy
Determining when to initiate therapy in patients with grade II gliomas is important. Many patients present with isolated epilepsy that is well controlled on antiepileptic medications or are only found to have a lesion incidentally after neuroimaging is performed for an unrelated indication (eg, trauma, etc). Patients with LGGs have a favorable prognosis and longer survival times than those with HGGs. Side effects from treatment are thus important considerations, and delayed neurocognitive dysfunction from RT or secondary leukemia following alkylating chemotherapy must be weighed against the benefits of treatment.
Historically, careful clinical and radiographic observation or “watchful waiting” was favored for many patients with grade II gliomas, including young, healthy patients with low-risk disease. In the late 1990s, the European Organisation for Research and Treatment of Cancer (EORTC) trial 22845 conducted a study to address the question of timing of treatment. A total of 314 patients were randomized to early or delayed RT; the investigators collected data on PFS, seizure control, and OS.[20] While there was improvement in PFS and seizure control at 1 year in the early RT group, OS was equivalent in the two study groups (median OS, 7.2 years with delayed RT vs 7.4 years with early RT). Given the potential side effects of treatment, these data strongly supported a general strategy of initiating treatment for patients with clinical symptoms, radiographic progression, or rare cases of refractory epilepsy. Surveillance was advocated in asymptomatic patients and those with favorable-risk disease. With the increasing use of MRI over the past 15 years, a number of clinical and radiographic risk factors associated with poorer prognosis have been identified, including age > 40 years, astrocytoma histology, largest tumor diameter > 6 cm, tumor crossing the midline, and preoperative focal neurologic deficit.[21] While it does appear that both tumor type (ie, histology and molecular markers) and tumor size are important prognostic factors, further study is needed to incorporate a reliable algorithm into the management of these tumors.[22]
The emerging data from RTOG 9802 do not address the question of timing of therapy, and at present there are no randomized prospective data to support early treatment in every patient with a newly diagnosed grade II glioma. Observation with serial examinations and imaging is reasonable for young, asymptomatic patients presenting with isolated epilepsy or an incidentally diagnosed lesion, and particularly for those with oligodendroglial pathology who have undergone gross total resection. Data support initiation of treatment when patients are symptomatic or have evidence of radiographic progression. In asymptomatic patients with large residual tumor or those with a nonprogressive lesion involving eloquent cortex, some have advocated early intervention; however, existing randomized prospective data support close observation and initiation of treatment in patients with clinical or radiographic progression.
Chemotherapy in 1p19q Codeleted Grade II Gliomas
Historically, management of treatment-naive LGGs involved RT without chemotherapy regardless of histopathologic or molecular characteristics. Advances in the treatment of HGGs over the past 10 to 15 years provide an important perspective on discussing optimal treatment for patients with grade II gliomas. In the last 2 years, data from two large prospective phase III studies evaluating the role of PCV in anaplastic oligodendroglial neoplasms (AOs) and anaplastic oligoastrocytoma (AOA); EORTC trial 26951, RTOG 9402) have highlighted the importance of genetic markers in treatment selection. In the EORTC 26951 study, 368 patients with newly diagnosed AO or AOA were randomized to RT alone or RT followed by 6 cycles of PCV.[6,23] While initial results in 2006 did not demonstrate a survival benefit, long-term follow-up did reveal a significant OS advantage to combination therapy (3.5 years vs 2.6 years in the RT-only arm; P = .018). This advantage was achieved after a median of 3 PCV cycles and with only 30% of patients completing all 6 cycles. Hematologic toxicity was the most common reason for discontinuation (33%), as well as nonhematologic toxicity (5%), patient refusal (5%), disease progression (24%), and other causes (4%). This study was one of the first to highlight the prognostic significance and chemosensitivity of 1p19q codeleted tumors. Patients with codeletion of 1p19q had significantly longer PFS and OS. This was observed regardless of treatment group, although a trend towards greater benefit in the combined RT-PCV treatment arm was observed (median survival not reached at 11.7 years in the PCV arm vs 9.3 years with RT only).
The simultaneously occurring RTOG 9402 study randomized 291 similar patients (with grade III gliomas) to RT alone or 4 cycles of neoadjuvant intensive PCV (high-dose lomustine, at 130 mg/m2) followed by RT.[7,24] Survival was not different in patients without 1p19q codeletion (2.6 years with PCV-RT vs 2.7 years with RT only) but was doubled in codeleted patients treated with PCV (14.7 years vs 7.3 years with RT only). Again, these data underscore the importance of using this marker to help optimize patient selection. Together these studies have led to widespread incorporation of PCV into treatment regimens for patients with 1p19q codeleted-but not non-codeleted-anaplastic gliomas. Additional subgroup analysis of these data has also suggested that IDH mutational status may also play a future role in treatment selection, with non-codeleted, IDH-mutated LGGs responding more favorably to chemoradiotherapy than non-codeleted, non-mutated tumors.[25]
Drawing from these two randomized prospective trials in patients with grade III glioma, similar chemosensitivity could be expected in patients with 1p19q codeleted grade II gliomas. While detailed molecular and immunohistochemical subgroup analysis is not yet available for the RTOG 9802 study, both treatment type and histology were independently associated with prognosis. As expected, pure oligodendrogliomas had better prognosis than mixed oligoastrocytomas (HR for OS = 2.06; P < .001), and both were better than astrocytomas (HR = 2.54; P < .001).[19] These findings strongly support the inclusion of PCV in treatment regimens for all 1p19q codeleted gliomas. Additional prospective study is necessary to determine whether IDH mutational status can be used routinely in treatment selection.
Despite this improvement in OS with the addition of PCV, toxicity often limits completion of the full regimen. Some studies have also raised questions regarding the necessity of certain agents (ie, vincristine) in this combination regimen.[26] Given these considerations, many investigators have sought to identify alternative treatments with more favorable side-effect profiles. TMZ is associated with less toxicity and improved compliance, and this agent generally is considered to be better tolerated than PCV. Small, single-arm, open-label phase II studies in newly diagnosed and recurrent AO have suggested favorable responses to TMZ monotherapy; however, large, randomized prospective studies have not yet been performed.[27,28] Similarly, several small, single-arm phase II studies have evaluated radiographic responses to TMZ in patients with progressive LGGs. All of these studies have been limited by small heterogeneous populations, lack of randomization, poor historical control data, and inherent limitations of radiographic evaluations in low-grade lesions with varying degrees of contrast enhancement.[29-32]
RTOG 0424 was a nonrandomized phase II study designed to further address the question of the utility of TMZ in chemotherapeutic management of LGGs. A total of 136 patients with LGGs and at least 3 high-risk features were treated with RT and concurrent TMZ followed by 6 to 12 cycles of adjuvant TMZ. Final results of this study have not yet been reported but were initially presented at the 2013 ASCO meeting. With a median follow-up of 4.1 years, the primary endpoint of 3-year OS was reported as 73.1%.[33] This was statistically significantly higher than that of reported historical controls (54%; P < .0001) and was interpreted by the authors as providing early data to support continued investigation of TMZ. To date, a large phase III investigation has not been completed. Categorization of response using genetic markers such as methylation status of the MGMT gene has not yet been reported for this patient population.
Accordingly, while future data may ultimately support TMZ as an alternative to PCV, the current body of literature provides strong evidence for the addition of PCV to RTfor patients with 1p19q codeleted grade II gliomas. Until sufficient prospective randomized data support an alternative approach, the 5.5-year survival advantage reported in the RTOG 9802 study provides strong evidence for the incorporation of PCV into the treatment plan.
Chemotherapy in Non-Codeleted Grade II Gliomas
Unlike 1p19q codeleted gliomas, non-codeleted tumors have been less responsive to chemotherapy. Many agents have been tried, including PCV, TMZ, and other chemotherapeutic combinations. While data from patients with high-grade oligodendrogliomas have helped to inform the inclusion of PCV in treatment regimens for codeleted LGGs, the response and survival data are much less impressive in patients with non-codeleted grade III gliomas.
In the 1990s, adjuvant PCV was commonly used in the management of malignant astrocytomas. Several small, prospective studies suggested improvement in survival compared to results in patients treated with alternative chemotherapies (eg, carmustine, or BCNU).[34] However, pooled retrospective meta-analyses of data from this era did not document a survival advantage to PCV over BCNU. In a rare randomized prospective phase III study conducted by the Medical Research Council in the United Kingdom, patients with glioblastoma multiforme (GBM) and anaplastic astrocytoma (AA) were randomized to RT alone or RT with adjuvant PCV for a total of 12 cycles (median, 3).[27] No significant difference in median survival was observed, and the authors concluded that data are lacking to support broad use of PCV in high-grade astrocytomas.[35]
Investigation then turned to using TMZ as adjuvant treatment in HGGs. In 2005, the landscape for treating GBM changed with the reporting of EORTC 22981 by Stupp and colleagues.[36,37] In this study, adult patients (18 to 70 years of age) with GBM were randomized to adjuvant RT alone or concurrent chemoradiation with TMZ followed by 6 cycles of monthly adjuvant TMZ. While this study did not meet its prespecified median survival goal (16 months), median survival did improve from 12.1 months to 14.6 months (combined treatment), and a substantial increase in the 2-year survival rate, from 10.4% to 26.5%, was observed. TMZ was well tolerated, with only slight increases in grade 3 or 4 neutropenia and thrombocytopenia. A total of 87% of patients completed all concurrent TMZ. A median of 3 cycles of adjuvant TMZ was achieved, with 47% of patients completing all 6 cycles. Few patients discontinued adjuvant TMZ due to toxicity (8%) or patient refusal (4%), and the majority of indications for stopping were disease progression (39%). Survival and response to treatment were significantly more favorable in patients whose tumors exhibited MGMT promoter methylation.[38]
Data on the efficacy of this treatment approach have not yet been replicated in studies of other types of astrocytomas. Currently available data in newly diagnosed AAs are entirely retrospective and have included reports of favorable and detrimental effects on survival in these patients.[39-41] Prospective studies are ongoing and will address the role of TMZ in AA. However, due to the favorable results in GBM, the lack of chemotherapeutic alternatives, and the poor survival in this population, inclusion of TMZ in regimens for AA has become common practice in the United States.[42]
Given the apparent lack of efficacy of PCV in grade III and IV astrocytomas, it is reasonable to anticipate that this regimen may not be effective in grade II astrocytoma. This will be clearer once further subgroup analysis from RTOG 9802 is performed. Extrapolating from data in high-grade astrocytomas, one might expect that TMZ would be more likely than PCV to be the optimal adjuvant chemotherapy in the general non-codeleted population. Results of the RTOG 0424 study have suggested that this may be the case; however, randomized data are lacking. A phase III study randomizing patients with symptomatic LGGs to RT alone or RT with TMZ was initiated through the Eastern Cooperative Oncology Group (ECOG E3F05, ClinicalTrials.gov ID NCT00978458). In light of the data from RTOG 9802, however, some have questioned whether a control arm of RT alone remains appropriate. Furthermore, clinicians are cognizant that care must be taken in extrapolating data from GBM to the clinical setting of LGG, given evidence that early treatment of LGGs with TMZ can induce new driver mutations and higher-grade malignancy at recurrence.[43]
In summary, the utility of chemotherapy in non-codeleted grade II gliomas remains unclear. These lesions appear to have a different biology and chemosensitivity than their 1p19q codeleted counterparts. Currently available randomized prospective data support RT alone as the treatment regimen of choice in these non-codeleted patients. Further study is required to determine the role of IDH mutational status in treatment selection for these patients.
Unresolved Questions Regarding Chemotherapy in Patients With Grade II Gliomas
Despite a growing body of data informing the chemotherapeutic management of grade II gliomas, several important questions remain. As previously described, greater extent of surgical resection (ie, GTR > STR > biopsy) has been repeatedly associated with longer survival. This finding, however, was not confirmed in early reports of RTOG 9802, although comprehensive subgroup analysis is not yet available. It may be that the robust chemosensitivity of 1p19q codeleted grade II gliomas outweighs the prognostic impact of the extent of surgery; however, we await further analysis from these data to guide considerations regarding the extent of surgery. This may prove particularly important for patients with progressive 1p19q codeleted LGGs. Given that data now support treatment of both grade II and grade III 1p19q codeleted gliomas with RT followed by PCV, repeat surgery may not be required to determine tumor grade.
The role of chemotherapy in delaying and deferring RT also remains controversial. EORTC 22033-26033 was a large phase III study that sought to address the question of utilizing chemotherapy to defer or delay RT. After molecular characterization of 1p status, 471 patients with LGGs were randomized to RT alone or initial TMZ monotherapy. While final results are not yet available, initial reports were presented at ASCO in 2013.[44] At a median follow-up of 45.5 months and after 246 patients (52%) had progressed, there was no statistically significant difference in PFS between the treatment arms. The authors concluded that additional maturation of these data was necessary but there were insufficient data to support deferring RT with upfront TMZ in patients with LGG. Furthermore, given the profound 5.5-year survival advantage observed with the combination of RT plus PCV in the RTOG 9802 study, deferring upfront RT poses the risk of losing a substantial survival advantage.
Conclusions
In conclusion, while the landscape for managing grade II gliomas is changing, existing data and experience in the management of high- and low-grade glial neoplasms continue to inform interpretation and integration of these data into standard practice. Based on currently available data, the following conclusions can be drawn:
(1) There are currently no randomized prospective data to support early treatment of all grade II gliomas, particularly in patients with well-controlled, isolated epilepsy or those whose lesion was identified incidentally on neuroimaging.
(2) There are currently no randomized prospective data to support delaying RT with chemotherapy monotherapy.
(3) There are strong randomized prospective data to support the addition of adjuvant PCV to RT in selected patients with grade II gliomas. Our current institutional practice is to incorporate PCV in patients with 1p19q codeleted grade II gliomas.
(4) There are currently no randomized prospective data to support adjuvant PCV in patients with non-codeleted grade II gliomas.
Several questions remain unresolved, including the appropriate timing of treatment; the role of chemotherapy in delaying RT; the role of IDH mutational status in treatment selection for non-codeleted LGGs; and the extent of surgery in LGGs, especially 1p19q codeleted tumors. Despite uncertainty in these areas of patient management, the treatment of grade II gliomas is changing, and the algorithmon on page 1038 (see Figure) utilizes currently available data from large prospective studies of grade II gliomas.
For young, asymptomatic patients with isolated epilepsy at presentation or an incidentally discovered grade II glioma, close follow-up with serial exams and neuroimaging is not unreasonable. Similarly, postoperative residual disease is not considered an absolute indication for immediate treatment and should be interpreted within the context of the clinical and radiographic trajectory of the patient. Data clearly show that patients with high-risk disease should begin early treatment. When treatment is initiated, strong randomized prospective data support the addition of PCV to RT in patients with codeleted grade II gliomas. There are no currently available data to support routine use of adjuvant PCV or TMZ in patients with non-codeleted tumors. The addition of TMZ could be considered on a case-by-case basis in select patients with symptomatic, progressive, or high-risk LGA and a concern about higher-grade features (ie, contrast enhancement, surgical sampling bias, etc). Because of its prognostic value, MGMT methylation has facilitated patient selection in the setting of HGGs, but its role in management of LGGs is still unclear.
In summary, it is encouraging to see recent progress in the chemotherapeutic management of codeleted grade II gliomas. Practitioners in the field await the final report from RTOG 9802 to help them determine how to integrate these findings into the management of non-codeleted grade II tumors. Ongoing investigation into alternative agents, including TMZ, for treatment of these neoplasms will be an important area of continued study.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Forst DA, Nahed BV, Loeffler JS, Batchelor TT. Low-grade gliomas. Oncologist. 2014;19:403-13.
2. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007;114:97-109.
3. Kim YH, Nobusawa S, Mittelbronn M, et al. Molecular classification of low-grade diffuse gliomas. Am J Pathol. 2010;177:2708-14.
4. Zhao J, Ma W, Zhao H. Loss of heterozygosity 1p/19q and survival in glioma: a meta-analysis. Neuro Oncol. 2014;16:103-12.
5. Boots-Sprenger SH, Sijben A, Rijnties J, et al. Significance of complete 1p/19q co-deletion, IDH1 mutation and MGMT promoter methylation in gliomas: use with caution. Mod Pathol. 2013;26:922-9.
6. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31:344-50.
7. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31:337-43.
8. Ohgaki H, Kleihues P. Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol. 2011;28:177-83.
9. Everhard S, Kaloshi G, Criniere E, et al. MGMT methylation: a marker of response to temozolomide in low-grade gliomas. Ann Neurol. 2006;60:740-3.
10. Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg. 2011;115:240-4.
11. McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63:700-7.
12. Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549-56.
13. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-45.
14. Ius T, Isola M, Budai R, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg. 2012;117:1039-52.
15. Maichrzak K, Kaspera W, Bobek-Billewicz B, et al. The assessment of prognostic factors in surgical treatment of low-grade gliomas: a prospective study. Clin Neurol Neurosurg. 2012;114:1135-44.
16. Koc K, Anik I, Cabuk B, Cevlan S. Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Br J Neurosurg. 2008;22:99-103.
17. Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267-76.
18. Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30:3065-70.
19. Buckner JC, Pugh SL, Shaw EG, et al. Phase III study of radiation therapy (RT) with or without procarbazine, CCNU, vincristine (PCV) in low-grade glioma: RTOG 9802 with Alliance, ECOG, SWOG. J Clin Oncol. 2014;32(suppl 5s):abstr 2000.
20. van den Bent MJ, Afra D, de Witte O, et al; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985-90.
21. Pignatti F, van den Bent M, Curren D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076-84.
22. Daniels TB, Brown PD, Felten SJ, et al. Validation of EORTC prognostic factors for adults with low-grade glioma: a report using Intergroup 86-72-51. Int J Radiat Oncol Biol Phys. 2011;81:218-24.
23. van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715-22.
24. Intergroup Radiation Therapy Oncology Group Trial 9402; Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707-14.
25. Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32:783-90.
26. Boyle FM, Eller SL, Grossman SA. Penetration of intra-arterially administered vincristine in experimental brain tumor. Neuro-oncol. 2004;6:300-5.
27. Mikkelsen T, Doyle T, Anderson J, et al. Temozolomide single-agent chemotherapy for newly diagnosed anaplastic oligodendroglioma. J Neurooncol. 2009;92:57-63.
28. Gan HK, Rosenthal MA, Dowling A, et al. A phase II trial of primary temozolomide in patients with grade III oligodendroglial brain tumors. Neuro Oncol. 2010;12:500-7.
29. Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133-8.
30. Quinn JA, Reardon DA, Friedman AH, et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol. 2003;21:646-51.
31. van den Bent MJ, Taphoorn MJ, Brandes AA, et al. Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol. 2003;21:2525-8.
32. Pace A, Vidiri A, Galiè E, et al. Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol. 2003;14:1722-6.
33. Fisher BJ, Lui J, Macdonald DR. A phase II study of a temozolomide-based chemoradiotherapy regimen for high-risk low-grade gliomas: preliminary results of RTOG 0424. J Clin Oncol. 2013;31(suppl):abstr 2008.
34. Levin VA, Silver P, Hannigan J, et al. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18:321-4.
35. Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19:509-18.
36. Stupp R, Mason WP, van den Bent MJ, et al; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-96.
37. Stupp R, Hegi ME, Mason WP, et al; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-66.
38. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003.
39. Shonka NA, Theeler B, Cahill D, et al. Outcomes for patients with anaplastic astrocytoma treated with chemoradiation, radiation therapy alone or radiation therapy followed by chemotherapy: a retrospective review within the era of temozolomide. J Neurooncol. 2013;113:305-11.
40. Scoccianti S, Magrini SM, Ricardi U, et al. Radiotherapy and temozolomide in anaplastic astrocytoma: a retrospective multicenter study by the Central Nervous System Study Group of AIRO (Italian Association of Radiation Oncology). Neuro Oncol. 2012;14:798-807.
41. Kizilbash SH, Giannini C, Voss JS, et al. The impact of concurrent temozolomide with adjuvant radiation and IDH mutation status among patients with anaplastic astrocytoma. J Neurooncol. 2014 Jul 4. [Epub ahead of print]
42. Holdhoff M, Grossman SA. Controversies in the adjuvant therapy of high-grade gliomas. Oncologist. 2011;16:351-8.
43. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189-93.
44. Baumert BG, Mason WP, Ryan G, et al. Temozolomide chemotherapy versus radiotherapy in molecularly characterized (1p loss) low-grade glioma: a randomized phase III Intergroup study by the EORTC/NCIC-CTG/TROG/MRC-CTU (EORTC 22033-26033). J Clin Oncol. 2013;31(suppl):abstr 2007.