(18)F-NaF PET/CT and (11)C-Choline PET/CT for the Initial Detection of Metastatic Disease in Prostate Cancer: Overview and Potential Utilization
We briefly review these two imaging technologies and provide potential utilization strategies based on available data.
Table: Recommendations From Various Organizations for the Use of Imaging to Detect Metastatic Disease in Prostate Cancer
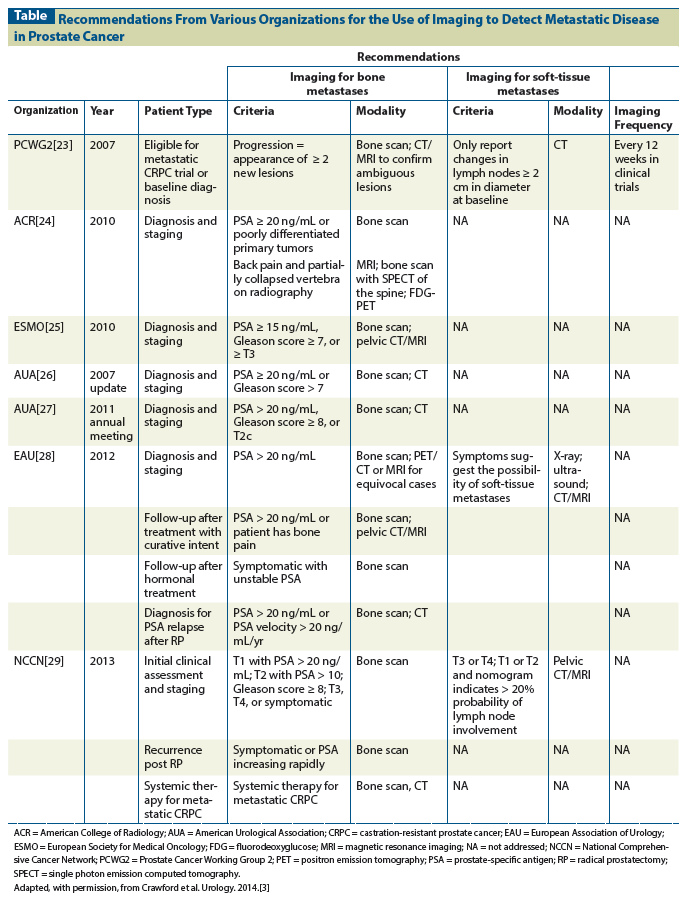
Figure 1: (18)F-NaF PET/CT in a 70-Year-Old Man With Biochemically Recurrent Prostate Cancer Who Presented With a PSA Level of 5.5 ng/mL
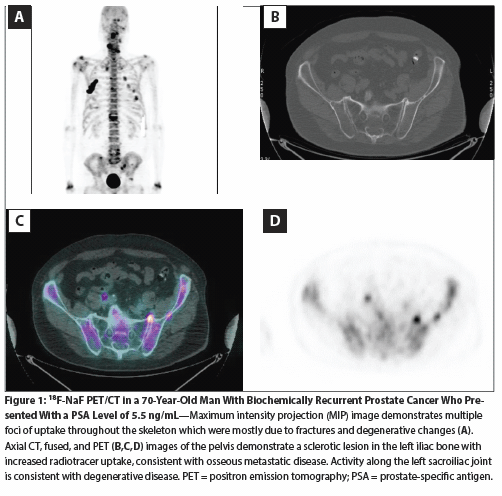
Figure 2: Potential Utilization Strategies for (18)F-NaF PET/CT and (11)C-choline PET/CT to Detect Advanced Disease in Different Patient Groups with Prostate Cancer
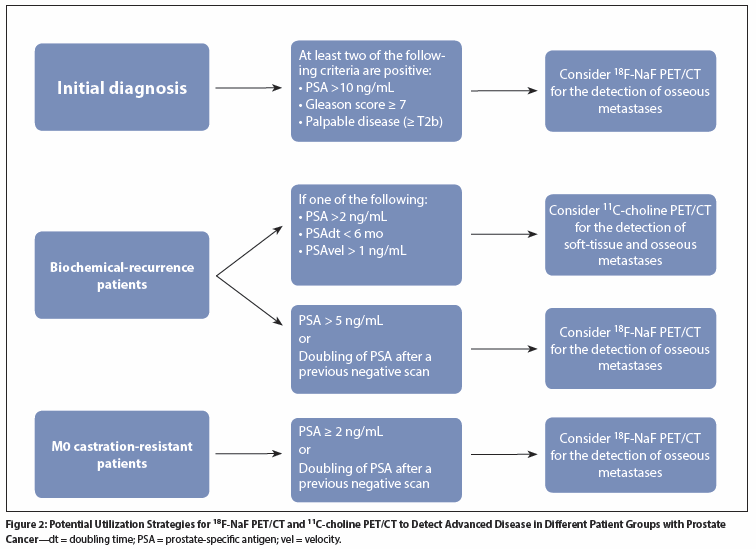
Figure 3: (11)C-Choline PET/CT in a 76-Year-Old Man With T2bN0M0, Gleason Score 7 Prostate Cancer
With the rapid increase in new therapies to treat advanced prostate cancer, improved diagnostic tools are necessary to help refine patient management throughout the entire disease course. Many radiopharmaceuticals, most of which are imaged using positron emission tomography/computed tomography (PET/CT), are in development; two of these newer radiopharmaceuticals are 18F-sodium fluoride (NaF) and radiolabeled choline. Compared with traditional imaging, use of 18F-NaF has been shown to improve sensitivity and specificity, and radiolabeled choline has been shown to detect recurrent and metastatic disease earlier. We briefly review these two imaging technologies and provide potential utilization strategies based on available data.
Introduction
New imaging technologies have been developed for the detection of advanced prostate cancer; however, the standard of care for imaging remains computed tomography (CT) and 99mTc-methylene diphosphonate (MDP) bone scanning. 99mTc-MDP bone scanning has been the workhorse in bone imaging for decades even though it is a two-dimensional, planar-based imaging technique. Three-dimensional imaging utilizing single photon emission computed tomography (SPECT) is often not used owing to time constraints and perceived lack of necessity. CT is an excellent technique for the evaluation of soft tissues, but it mainly provides an anatomic/morphologic assessment of disease, which is often a later manifestation of the presence of malignancy. The imaging and oncology communities consider these techniques to be inadequate. With the many new therapies now offered to treat advanced prostate cancer, it is imperative that diagnostic tools be available to provide more accurate data as early as possible so that therapeutic management can be optimized throughout the entire disease course.
There have been many new and exciting developments in the area of prostate cancer imaging. In contrast to many other malignancies, however, prostate cancer has not yet benefited from 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT. In a study looking at biochemical recurrence in 37 men with prostate cancer, FDG PET/CT showed very limited utility.[1] For the detection of metastatic disease, PET/CT would be the ideal imaging modality as it allows for whole-body imaging and for the combination of physiologic and anatomic evaluation of disease. With PET/CT, the PET examination captures the physiologic aspects of disease that can be shown by the type of radiopharmaceutical administered, and the CT examination provides attenuation correction and activity localization, and allows for morphologic assessment of disease. Semiquantitative analysis of PET/CT is also more robust than other nuclear imaging technologies. The main drawback of PET is limited resolution (in the range of 3 to 5 mm); however, PET is superior to other nuclear imaging technologies, and new hardware and software developments continue to improve it. Although whole-body imaging is also available with magnetic resonance imaging (MRI), it is likely that MRI will have its greatest impact in evaluating local disease and local recurrence.
The radiopharmaceuticals 18F-sodium fluoride (NaF) and 11C-choline are available for clinical use in the United States, although the latter has limited accessibility. 18F-NaF and radiolabeled choline have demonstrated promising results when used with PET/CT, but mostly in single-site, retrospective studies with limited numbers of patients. It is challenging to obtain outcome data, the desired goal of clinical trials, when evaluating diagnostic procedures. The true effect of the examination results is difficult to accurately analyze, given the numerous downstream variables, including additional imaging and variations in therapeutic management. In 2012, the Medical Imaging & Technology Alliance conference convened a group of experts and stakeholders to discuss possible research endpoints that might be used to demonstrate that new PET procedures and radiopharmaceuticals lead to improved outcomes. The attendees agreed that intermediate endpoints should be used to generate data to support coverage decisions by the Centers for Medicare and Medicaid Services (CMS).[2]
The development of metastases is a seminal event in the progression of prostate cancer. As seen in the Table, there is no consensus in the literature regarding preferred imaging modality and frequency of scanning to detect advanced disease.[3] Evidence-based recommendations for imaging exist for initial diagnosis and continue to evolve. In multivariate analyses, Merdan et al reported that the prostate-specific antigen (PSA) level and Gleason score were the only independent predictors of a positive bone scan. They also noted that scanning only patients with PSA > 20 ng/mL or Gleason score ≥ 8 would miss < 1% of patients with bone metastases and would reduce the number of negative scan results by 38%.[4] Such evidence-based recommendations are lacking for patients with biochemical recurrence and castration-resistant prostate cancer. It is premature to incorporate advanced imaging tools into routine clinical practice; nevertheless, compelling data regarding their ability to detect recurrent/metastatic bone and soft-tissue disease more accurately and earlier than traditional technologies will likely lead to eventual changes in guidelines. Despite the lack of sufficient data supporting the routine use of these advanced imaging techniques, their availability sparks many questions from patients and clinical colleagues. To help address some of these questions, we will briefly review these agents with respect to their ability to detect metastatic disease in prostate cancer. Then, based on existing data, we will discuss cost considerations and possible implementation strategies for clinical practice.
18F-NaF PET/CT
18F-NaF was first approved by the US Food and Drug Administration (FDA) in 1972. We have seen resurgent interest in it over the past decade, in part due to the increasing availability of PET/CT scanners across the country. As discussed above, the use of PET/CT in bone imaging presents several technical advantages over traditional bone scanning, but costs remain a significant concern. 18F-NaF is rapidly cleared from the blood, which allows for a shorter time interval between injection and imaging.[5] This in turn allows for a much shorter overall imaging time compared with conventional 99mTc-based bone scans, which makes the procedure more convenient for patients. Radiation exposure from the radiopharmaceutical alone is similar to that from 99mTc-based bone scan agents.[6] However, the total radiation dose is greater than that from traditional bone scanning because of the CT portion of the PET/CT examination.
In a prospective study of 44 patients (23 of whom had bone metastases based on PET/CT findings, biopsy, and imaging follow-up), sensitivity, specificity, positive predictive value, and negative predictive value were as follows: 99mTc planar bone scan, 70%, 57%, 64%, and 55%; SPECT, 92%, 82%, 86%, and 90%; 18F-NaF PET, 100%, 62%, 74%, and 100%; and 18F-NaF PET/CT 100%, 100%, 100%, and 100%, respectively.[7] A meta-analysis from Tateishi et al demonstrated similar results after reviewing 11 studies and a total of 425 patients.[8] The pooled sensitivity and specificity for 18F-NaF PET and 18F-NaF PET/CT on a patient basis were 96.2% and 98.5%, respectively. The area under the curve, based on the summary receiver operating characteristic curve analysis, was 0.986 for 18F-NaF PET and PET/CT vs 0.866 for bone scan and SPECT.
The National Oncologic PET Registry (NOPR) began accrual of 18F-NaF PET/CT data in January 2011. NOPR was created in consultation with CMS to evaluate PET's impact on intended management, using prospective questionnaire data before and after PET studies. Louis Jacques, MD, then Director of the Coverage and Analysis Group at CMS, reported a willingness to consider intermediate endpoints, such as changes in management, that might improve health outcomes.[2] Initial NOPR results were recently published based on a dataset of 3,531 scans performed on 3,396 prostate cancer patients referred for initial staging, suspected first osseous metastasis in men with previously treated local disease, and suspected progression of osseous metastasis. Intended management (treatment vs nontreatment) changed in 44% to 52% of cases after 18F-NaF PET/CT was performed. When taking into consideration the likelihood that pre-18F-NaF PET/CT plans would have led to the same decision, the impact of 18F-NaF was still significant at 12.2% to 15.8%.[9] No data were available for 18F-NaF PET/CT performed after 99mTc bone scanning. Hillner et al concluded that 18F-NaF PET/CT had a significant impact across common imaging indications in prostate cancer.[9]
Yet, despite such data, false-positive scans remain a significant concern. The high number of false-positives is likely due to the steep learning curve associated with interpretation of this sensitive examination. Increased activity is seen in areas of increased perfusion and bone matrix, which would include both malignant and benign processes. The exam's specificity is increased through careful evaluation of the CT component to characterize areas of uptake on PET as either malignant or benign (eg, having etiologies such as degenerative disease or trauma).[7] Figure 1 demonstrates multiple foci of uptake that were for the most part due to post-traumatic and degenerative changes; however, there was a single focus of uptake in the left iliac bone that was consistent with an osseous metastasis. Adjacent activity in the sacroiliac joint, by contrast, was due to degenerative disease, which was confirmed on the CT component of the study.
Cost considerations
According to the initial results from the NOPR, high-cost treatment plans declined by more than 50% after 18F-NaF PET results. Additionally, physicians reported that 70% to 80% of patients would avoid additional imaging and that biopsy or additional imaging would be necessary in only 7% to 12% of cases.[9] Based on these preliminary results, the authors concluded that the principal impact of 18F-NaF PET/CT will be in replacing other advanced imaging techniques. We await the final NOPR results to gain greater insight into its impact on costs.
18F-NaF is widely available from most commercial radiopharmacies, and the price is similar to the more widely used FDG. Although the cost of PET/CT is greater than the cost of traditional gamma/SPECT imaging, there are potential cost savings because an optimized CT scan acquired in PET/CT could preclude the need for additional CT imaging for the evaluation of soft-tissue metastases.
Practical utilization
Currently, 18F-NaF PET/CT is covered by Medicare through the NOPR Continuing Evidence Development (CED) program. Further results from this registry will help guide the appropriate indications for 18F-NaF PET/CT by identifying the subgroup of patients who would most benefit from it. Additionally, the American College of Radiology is currently sponsoring a randomized, multicenter trial comparing the diagnostic performance of 18F-NaF PET/CT with that of traditional bone scanning, with a goal of helping determine whether the use of 18F-NaF PET/CT will lead to improved outcomes.[10] These studies will provide the pivotal data needed to guide the appropriate use of 18F-NaF PET/CT in clinical practice.
Based on available limited data and using the recommendations from the Prostate Cancer Radiographic Assessments for Detection of Advanced Recurrence (RADAR) Working Group, 18F-NaF PET/CT might be considered for the detection of advanced disease in the following clinical scenarios (but is not limited to these) (Figure 2)[3]:
In newly diagnosed patients with at least two of the following criteria:
•PSA level > 10 ng/mL.
•Gleason score ≥ 7.
•Palpable disease (≥ T2b).
In patients with biochemical recurrence:
•Initial scan when PSA level is > 5 ng/mL.
OR
•Doubling of PSA level after a negative prior scan.
In patients with M0 castration-resistant disease:
•Initial scan when PSA level is ≥ 2 ng/mL.
OR
•Doubling of PSA level after a negative prior scan.
Radiolabeled Choline PET/CT
Radiolabeled choline PET/CT has been investigated throughout the spectrum of prostate cancer. This technique allows for the detection of soft-tissue and bone metastases in a single scan (Figure 3). The most promising results have been for the detection of metastatic disease in patients with biochemical recurrence. In a series of 49 patients, Schillaci et al reported disease detection rates using 18F-choline PET/CT of 20% at a PSA value of ≤ 1 ng/mL, 55% at 1 to ≤ 2 ng/mL, 80% at 2 to ≤ 4 ng/mL, and 87% at > 4 ng/mL.[11] In patients with local relapse, biopsy of the prostatic fossa confirmed PET/CT results. In patients with systemic disease, findings were confirmed with additional imaging. A meta-analysis reviewing 11C-choline and 18F-fluorocholine in the setting of biochemical failure showed a pooled sensitivity of 85% and a pooled specificity of 88% in 637 patients.[12] Umbehr et al concluded that choline imaging may help guide further treatment decisions in patients with biochemical failure after local therapy.[12] A meta-analysis from Evangelista et al, which included 19 studies and 1,555 patients, demonstrated a pooled sensitivity and specificity of 86% and 93%, respectively, for all sites of disease.[13]
For the detection of bone metastases in the spine, Poulsen et al report the superiority of 18F-fluorocholine PET/CT over traditional bone scans.[14] Sensitivity and specificity for traditional bone scans were 51% and 82%, compared with 85% and 91% for 18F-fluorocholine PET/CT. Another study suggested that 18F-fluorocholine PET/CT may be superior to 18F-NaF PET/CT for the detection of early metastatic disease involving the bone marrow.[15] In this study of 38 patients, 18F-NaF PET/CT detected more lesions in some patients than did 18F-fluorocholine PET/CT, but it did not lead to a change in management. 18F-fluorocholine PET/CT led to a change in management in two patients, based on detection of early marrow disease. Final diagnosis was based on histology or follow-up examinations. Other studies, including a prospective study of detection of bone metastases in 42 prostate cancer patients, have shown that the performances of 18F-fluorocholine PET/CT and 18F-NaF PET/CT are similar.[16]
There are also concerns regarding the accuracy of 11C-choline. False-positive results with radiolabeled choline have occurred, mostly secondary to inflammation. Radiotracer uptake in non-prostate cancer-related malignancies is also a possibility. There can be technical limitations based on the limited resolution of PET/CT. Passoni et al report that in those with a single lesion on PET/CT, the concordance rate with pathology results was only 35%.[17] PET/CT was able to detect the exact site of metastasis in 30% of the patients; however, there was pathologic evidence of disease at other sites where it was not seen during imaging. Around 50% of the patients in this series had positive lymphatic stations at surgery that were not detected on the PET/CT scan. The authors conclude that a negative scan does not preclude the presence of micrometastatic disease, which is not surprising given the technical limitations of PET/CT. This may restrict the use of PET/CT imaging in planning therapy with targeted agents; however, the earlier detection of metastatic disease does allow for more appropriate initiation of systemic therapies, such as immunotherapy, for patients whose disease can now be classified as M1.
The impact of androgen deprivation therapy (ADT) prior to choline PET/CT scanning remains unclear. Fuccio et al found that ADT preceding choline PET/CT significantly decreased the detection capability of the study, on account of lower choline uptake in tumorous cells that have been deprived of androgen.[18] Another study concluded that hormonal treatment had no effect on PET/CT detection ability.[19]
Cost considerations
Imaging with 11C-choline is a high-cost procedure largely because of the short half-life of this agent and the requirement that it be produced at an on-site medical cyclotron facility. If 18F-fluorocholine were to become widely available, the cost of this study would be reduced, since the longer half-life of the latter radiopharmaceutical would allow it to be manufactured and distributed through the existing network of commercial radiopharmacies throughout the United States.
Because choline PET/CT can detect both bone and soft-tissue metastases with a single examination, cost savings might potentially be achieved by using it to replace the two separate studies traditionally ordered to evaluate bones and soft tissues.
Practical utilization
In 2012, 11C-choline was approved by the FDA for production and use at the Mayo Clinic. Its availability across the United States is still very limited, and reimbursement remains an issue. Institutions interested in 11C-choline for clinical use will need to obtain independent FDA approval to manufacture this PET radiopharmaceutical at their own facility.
The literature regarding radiolabeled choline continues to grow; however, most studies remain single-site and involve limited numbers of patients. We await more robust trials with data on changes in management and meaningful health-related outcomes. Based on existing data, 11C-choline PET/CT may be of benefit, if available, in patients with biochemical recurrence when salvage therapies are being considered. PSA levels and kinetics have been shown to correlate with choline PET/CT. Based on their review of the literature, Castellucci et al recommend considering choline PET/CT as the first-line diagnostic procedure in patients with biochemical relapse and PSA velocity > 1 ng/mL/yr or PSA doubling time < 6 months.[20] Others recommend its use in patients with PSA levels > 2 ng/mL, PSA doubling time ≤ 6 months, or PSA velocity > 2 ng/mL/yr, which would likely result in a higher percentage of positive results.[11] The recommendation we propose for the use of 11C-choline is shown in Figure 2.
Other Radiopharmaceuticals
There are many additional PET radiopharmaceuticals in development that have the potential to detect early metastatic disease in prostate cancer patients; however, these are currently only available in the research setting. 11C-acetate has been shown to have a performance similar to that of 18F-fluorocholine for the detection of recurrent prostate cancer.[21] Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (FACBC), which is a synthetic amino acid analog originally developed at Emory University, is also promising. Preliminary results have shown that this agent might be superior to 11C-choline in patients with biochemically recurrent disease.[22] A new start-up, Blue Earth Diagnostics, is focusing on the development and commercialization of FACBC in recurrent prostate cancer. Anti-prostate-specific membrane antigen agents are another new and exciting area of research; they have the potential not only to detect disease, but also to allow for targeted radiotherapy.
Conclusion
Hybrid imaging with PET/CT allows for physiologic and anatomic evaluation of prostate cancer. 18F-NaF and radiolabeled choline are tools that allow us to diagnose metastatic disease more accurately and earlier compared with traditional imaging. Costs and availability are significant barriers to acceptance, particularly for radiolabeled choline and other radiopharmaceuticals in development. These new tests will undoubtedly change the imaging of prostate cancer; however, further research is needed to evaluate meaningful health-related outcomes and to better identify the clinical scenarios in which these more expensive tests will have their greatest value. Studies are currently underway to help answer many of these questions, and we look forward to the results to help us optimize the management of patients with prostate cancer.
Financial Disclosure: Dr. Crawford serves as a consultant/advisor to Bayer, Dendreon, Ferring, Genomic Health, Janssen and MDx; he also serves as a lecturer for Ferring, and his wife is employed by Ferring. Dr. Koo has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Jadvar H, Desai B, Ji L, et al. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637-43.
2. Hillman BJ, Frank RA, Abraham BC. The Medical Imaging & Technology Alliance conference on research endpoints appropriate for Medicare coverage of new PET radiopharmaceuticals. J Nucl Med. 2013;54:1675-9.
3. Crawford ED, Stone NN, Yu EY, et al. Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology. 2014;83:664-9.
4. Merdan S, Womble PR, Miller DC, et al. Toward better use of bone scans among men with early-stage prostate cancer. Urology. 2014;84:793-8.
5. Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. 2010;51:1826-9.
6. Grant FD, Fahey FH, Packard AB, et al. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68-78.
7. Even-Sapir E, Metser U, Mishani E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287-97.
8. Tateishi U, Morita S, Taguri M, et al. A meta-analysis of (18)F-fluoride positron emission tomography for assessment of metastatic bone tumor. Ann Nucl Med. 2010;24:523-31.
9. Hillner BE, Siegel BA, Hanna L, et al. Impact of 18F-fluoride PET in patients with known prostate cancer: initial results from the National Oncologic PET Registry. J Nucl Med. 2014;55:574-81.
10. F18PET/CT versus TC-MDP scanning to detect bone mets. Available from: http://clinicaltrials.gov/ct2/show/NCT00882609?term=sodium+fluoride+PET%26phase=2%26rank=2. Accessed November 11, 2014.
11. Schillaci O, Calabria F, Tavolozza M, et al. Influence of PSA, PSA velocity and PSA doubling time on contrast-enhanced 18F-choline PET/CT detection rate in patients with rising PSA after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2012;39:589-96.
12. Umbehr MH, Muntener M, Hany T, et al. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64:106-17.
13. Evangelista L, Zattoni F, Guttilla A, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38:305-14.
14. Poulsen MH, Petersen H, Hoilund-Carlsen PF, et al. Spine metastases in prostate cancer: comparison of technetium-99m-MDP whole-body bone scintigraphy, 18F choline positron emission tomography (PET)/computed tomography (CT), and 18F NaF PET/CT. BJU Int. Epub 2013 Dec 9.
15. Beheshti M, Vali R, Waldenberger P, et al. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and18F fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging. 2008;35:1766-74.
16. Langsteger W, Balogova S, Huchet V, et al. Fluorocholine (18F) and sodium fluoride (18F) PET/CT in the detection of prostate cancer: prospective comparison of diagnostic performance determined by masked reading. Q J Nucl Med Mol Imaging. 2011;55:448-57.
17. Passoni NM, Suardi N, Abdollah F, et al. Utility of [11C]choline PET/CT in guiding lesion-targeted salvage therapies in patients with prostate cancer recurrence localized to a single lymph node at imaging: results from a pathologically validated series. Urol Oncol. 2014;32:38.e9-16.
18. Fuccio C, Schiavina R, Castellucci P, et al. Androgen deprivation therapy influences the uptake of 11C-choline in patients with recurrent prostate cancer: the preliminary results of a sequential PET/CT study. Eur J Nucl Med Mol Imaging. 2011;38:1985-9.
19. Osmonov DK, Heimann D, Jansen I, et al. Sensitivity and specificity of PET/CT regarding the detection of lymph node metastases in prostate cancer recurrence. SpringerPlus. 2014;3:340.
20. Castellucci P, Piccio M. 11C-choline PET/CT and PSA kinetics. Eur J Nucl Med Mol Imaging. 2013;40(suppl 1):S36-40.
21. Buchegger F, Garibotto V, Zilli T, et al. First imaging results of an intraindividual comparison of (11)C-acetate and (18)F-fluorocholine PET/CT in patients with prostate cancer at early biochemical first or second relapse after prostatectomy or radiotherapy. Eur J Nucl Med Mol Imaging. 2014;41:68-78.
22. Nanni C, Schiavina R, Brunocilla E, et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: a prospective study in 28 patients. Clin Genitourin Cancer. 2014;12:106-10.
23. Scher HI, Halabi S, Tannock I, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148-59.
24. Roberts CC, Daffner RH, Weissman BN, et al. ACR appropriateness criteria on metastatic bone disease.
J Am Coll Radiol. 2010;7: 400-9.
25. Horwich A, Parker C, Bangma C, et al; ESMO Guidelines Working Group. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v129-33.
26. American Urological Association. Prostate cancer guideline for the management of clinically localized prostate cancer: 2007 update. Available from: http://www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer.pdf. Accessed July 26, 2013.
27. Kazzazi A, Momtahen S, Bruhn A, et al. New findings in localized and advanced prostate cancer: AUA 2011 review. Can J Urol. 2011;18:5683-8.
28. European Association of Urology. Guidelines on prostate cancer. Available from: http://www.uroweb.org/gls/pdf/08%20Prostate%20Cancer_LR%20March%2013th%202012.pdf. Accessed July 26, 2013.
29. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer. Version 1.2013. Available from: http://www.nccn.org. Accessed November 14, 2014.
30. Castellucci P, Ceci F, Graziani T, et al. Early biochemical relapse after radical prostatectomy: which prostate cancer patients may benefit from a restaging 11C-choline PET/CT scan before salvage radiation therapy? J Nucl Med. 2014;55:1424-9.