Improving Outcomes in Advanced DLBCL: Systemic Approaches and Radiotherapy
In this review, we will first briefly summarize prior attempts to improve outcomes in advanced DLBCL using systemic therapy approaches, and then we will highlight the potential role of RT in advanced DLBCL.
Figure 1: Case Example for Advanced Stage DLBCL With a Limited Number of Involved Sites
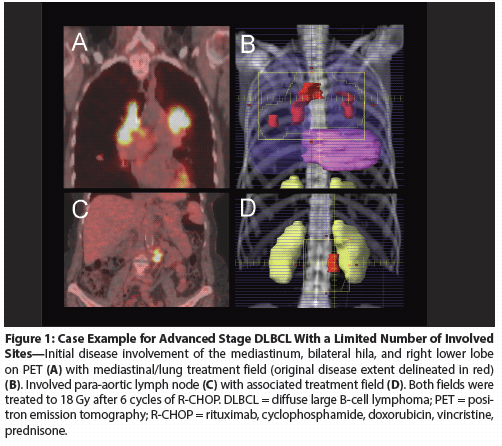
Figure 2: Case Example for Advanced-Stage, Bulky Disease in DLBCL
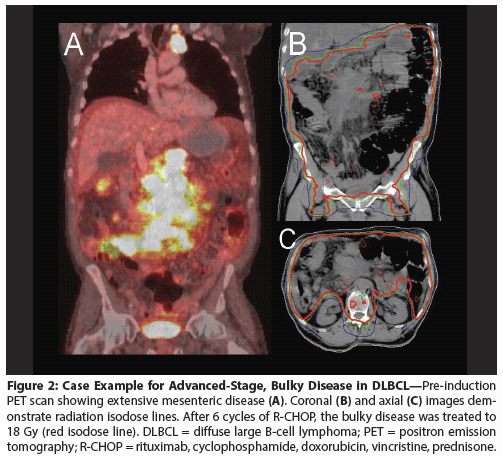
Figure 3: Case Example for Advanced-Stage DLBCL With Skeletal Involvement
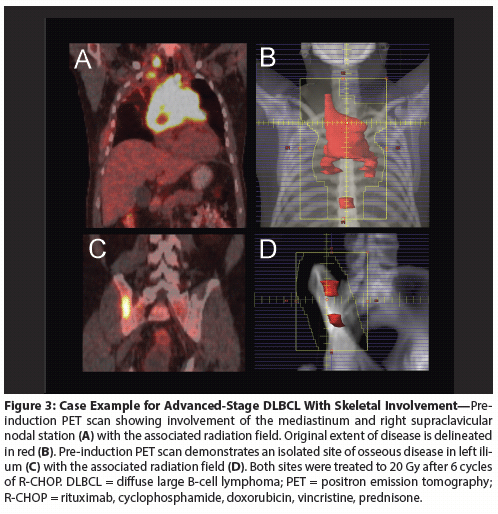
Figure 4: Case Example of a Partial Response to Induction Therapy in DLBCL
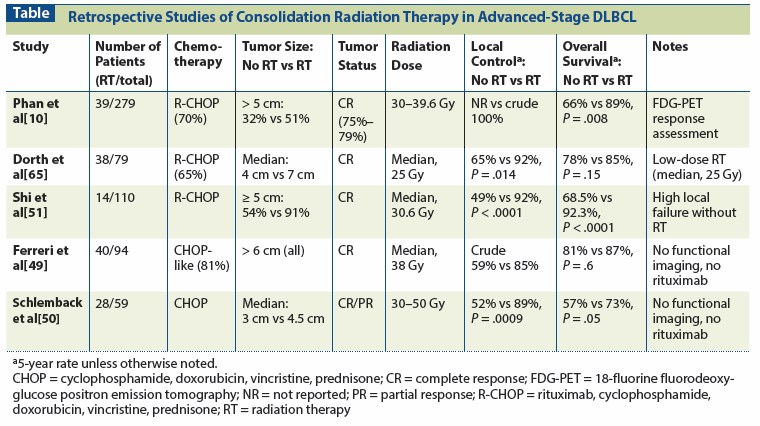
Table: Retrospective Studies of Consolidation Radiation Therapy in Advanced-Stage DLBCL
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma. Approximately half of patients will present with advanced (stage III/IV) disease. The cornerstone of treatment is a combination of chemotherapy and immunotherapy, most commonly R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). Efforts to improve upon R-CHOP-including more chemotherapy cycles, dose-dense chemotherapy, alternative drug combinations, high-dose chemotherapy with autologous stem cell transplant, and maintenance rituximab-have generally proved unsuccessful. There is a growing body of retrospective and prospective data, however, suggesting a benefit for consolidation radiation therapy (RT) in select patients with advanced DLBCL. Consolidation RT has been shown to improve outcomes for patients with advanced DLBCL generally, and in specific instances including initially bulky disease, bone involvement, or in the setting of a partial response to systemic therapy. In these settings consolidation RT is highly efficacious at achieving local disease control and improving overall outcomes.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), comprising 30% to 40% of all cases.[1,2] More than 20,000 patients are diagnosed with DLBCL each year in the United States. Approximately 50% of such patients present with stage I/II disease. As with many lymphomas, radiation therapy (RT) was historically the cornerstone of treatment for localized disease, achieving high complete response (CR) rates and long-term freedom from relapse in 40% to 45% of patients.[3-6] Over time, treatment evolved to incorporate chemotherapy[4,5,7,8] and rituximab,[9,10] both of which led to lower relapse rates and improved survival. Radiation remains an integral part of the treatment of localized disease, having been shown to decrease the risk of relapse after chemotherapy,[9-12] and it is currently designated as a category 1 treatment option by the National Comprehensive Cancer Network.[13] Now, 80% to 90% of patients with early-stage DLBCL remain disease-free after treatment with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) and consolidation RT.[9,10]
The other half of patients diagosed with DLBCL in the United States present with advanced disease managed primarily with systemic therapy. Stage is an important prognostic factor in DLBCL.[14] Patients with advanced disease have inferior clinical outcomes compared with those with localized presentations. In this review, we will first briefly summarize prior attempts to improve outcomes in advanced DLBCL using systemic therapy approaches, and then we will highlight the potential role of RT in advanced DLBCL.
Systemic Approaches
The primary modality for advanced-stage DLBCL is combination chemoimmunotherapy, now most commonly R-CHOP. A variety of treatment approaches have been explored in an attempt to improve outcomes. Phase II and randomized trials have evaluated different strategies, including delivery of more cycles of chemotherapy,[15] dose-dense chemotherapy,[16,17] alternative drug combinations,[18-25] high-dose chemotherapy followed by autologous stem cell transplant (HDT/ASCT),[26] and optimal incorporation of immunotherapy.[27,28] Only the addition of rituximab to standard CHOP chemotherapy has led to consistent and meaningful improvements in outcomes for patients with advanced DLBCL.[29]
R-CHOP
Perhaps no treatment to date has had a more profound impact on the prognosis of patients with advanced DLBCL than the incorporation of rituximab into combination chemotherapy regimens. The first study to enroll patients with advanced DLBCL was the LNH-98.5 trial conducted by the Groupe d’Etude des Lymphomes de l’Adulte (GELA).[27] LNH-98.5 randomized elderly patients with stage II–IV DLBCL to 8 cycles of CHOP or 8 cycles of R-CHOP chemotherapy. The addition of rituximab resulted in significantly higher rates of CR, progression-free survival (PFS), and overall survival (OS). At 10 years, PFS and OS were 36.5% and 43.5%, respectively, with R-CHOP compared with 20% and 27.6%, respectively, with CHOP.[30]
In a separate study, the MabThera International Trial (MinT) Group enrolled young patients (18 to 60 years of age) with stage II–IV DLBCL or stage I with bulky disease
(> 5 cm).[28] Patients were randomized to 6 cycles of CHOP-like chemotherapy with or without rituximab. RT was delivered to initial sites of bulky disease (at a dose of 30–40 Gy). Rituximab improved outcomes, with a longer 6-year PFS (81% vs 64%; P < .0001) and OS (86% vs 80%; P = .046).
Maintenance rituximab
The success of rituximab prompted the Eastern Cooperative Oncology Group (ECOG; trial 4494) and Cancer and Leukemia Group B (CALGB; trial 9793) to conduct a joint trial to test the efficacy of maintenance rituximab as a means to prevent relapse among patients who initially responded to induction therapy.[31] In this two-stage randomized study, patients were randomized first to CHOP or R-CHOP, with responders subsequently randomized to maintenance rituximab or observation. Although there was an improvement in rates of failure-free survival with maintenance rituximab, this was only apparent in patients who did not receive upfront rituximab. Thus, when rituximab is incorporated into the induction regimen, maintenance rituximab does not appear to confer an advantage.
Alternative drug combinations
In the pre-rituximab era, more “intense” chemotherapy regimens were developed and tested in single-arm, single-institution studies. OS rates were 62% for M-BACOD (methotrexate, bleomycin, doxorubicin, cyclo-phosphamide, vincristine, dexamethasone),[23] 69% for ProMACE-CytaBOM (prednisone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, methotrexate),[24] and 76% for MACOP-B (methotrexate with leucovorin rescue, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin),[25] whereas CHOP was considered to confer OS rates in the 30% range. SWOG and ECOG conducted a randomized study of these three regimens compared with CHOP, reporting OS rates of 50% for patients treated with ProMACE-CytaBOM and MACOP-B, 52% for those treated with M-BACOD, and 54% for patients who received CHOP.
One exception was a regimen consisting of doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACVBP) with additional consolidation chemotherapy.[21,22] Rituximab was subsequently added to this regimen (R-ACVBP) and compared against standard R-CHOP-21 in a recently reported French trial, GELA LNH03-2B.[32] While this regimen improved 3-year PFS (87% vs 73%; P = .0015) and OS (92% vs 84%; P = .0071), it has not been adopted into routine clinical practice out of concern about excess toxicity and increasing interest in alternative therapies such as DA-EPOCH-R (rituximab, dose-adjusted continuous infusion doxorubicin, vincristine, and etoposide with bolus cyclophosphamide and oral prednisone). A randomized study by the CALGB (50303) is currently testing R-CHOP vs DA-EPOCH-R in untreated DLBCL.
Dose-dense chemotherapy
Another strategy that has been investigated is dose-dense chemotherapy, in which the same total doses of chemotherapy are given in a shorter time period. In the pre-rituximab era, the German High-Grade Non-Hodgkin Lymphoma Study Group (Deutsche Studiengruppe Hochmaligne Non-Hodgkin Lymphome [DSHNHL]) demonstrated that administration of 6 cycles of CHOP every 2 weeks (CHOP-14, OS 50.3%) was superior to administration every 3 weeks (CHOP-21, OS 40.6%).[33] A subsequent study by GELA compared 6 cycles of dose-dense R-CHOP-14 with 6 cycles of R-CHOP-21.[17] There was no improvement in treatment efficacy (OS was 69% for patients treated with R-CHOP-14 and 72% for those randomized to CHOP-21). At the same time a UK study compared 6 cycles of dose-dense R-CHOP-14 vs 8 cycles of R-CHOP-21, again finding dose-dense therapy conferred no benefit.[16]
More chemotherapy cycles
As rituximab-containing regimens emerged as the standard of care, an interest arose in determining the optimal treatment schedule. The DSHNHL conducted the RICOVER-60 (Rituximab With CHOP Over 60 Years) trial. This study was designed to build upon the prior study which showed a benefit for treatment with dose-dense CHOP (CHOP-14). The study was conducted to ascertain both the benefit of rituximab added to CHOP-14 and the optimal number of chemotherapy cycles. In a 2 × 2 factorial design, patients were randomized to CHOP-14 vs R-CHOP-14, and then to either 6 or 8 cycles of chemotherapy. The 3-year OS rates were 67.7% for patients treated with 6-CHOP-14, 66.0% for those randomized to 8-CHOP-14, 78% for patients who received 6-R-CHOP-14, and 72.1% for those treated with 8-R-CHOP-14. The results of RICOVER-60 not only verified the superiority of rituximab-containing regimens but they also demonstrated no additional benefit for 8 cycles vs 6 cycles of R-CHOP-14. More treatment-associated deaths (8% vs 5.6%) and second malignancies (5% vs 2%) with 8 cycles vs 6 cycles likely contributed to the observed outcomes.
High-dose chemotherapy and autologous stem cell transplant
HDT/ASCT is an effective treatment for patients with relapsed disease.[34] Although initial results of pilot studies employing this technique as a first-line treatment strategy were promising,[35-37] subsequent randomized trials showed no significant survival benefit.[38-40] A recent meta-analysis found that, compared with conventional chemotherapy, HDT/ASCT offers no benefit in terms of OS or event-free survival (EFS), regardless of the patient’s International Prognostic Index (IPI) risk group.[26]
A recently reported randomized trial, SWOG 97-04, investigated consolidation ASCT following induction using CHOP or R-CHOP.[41] Unlike the meta-analysis described above, this study employed rituximab in 253 patients. While there was an improvement in PFS, this did not translate into an OS benefit. This was due, at least in part, to treatment-related deaths. An unplanned subset analysis did show a benefit of consolidation ASCT in terms of both PFS and OS in the high-risk IPI group. However, a larger study of ASCT in high-risk patients treated with rituximab-based regimens will be needed before this becomes a standard part of first-line therapy.
Summary of approaches to systemic therapy
Many modifications of systemic therapy have been explored in an attempt to improve outcomes in patients with advanced DLBCL. With the exception of immunotherapy with rituximab, the other attempts have been proven to be largely ineffective. Rituximab-based strategies seem to have hit a ceiling, with efforts at dose intensification and maintenance therapy failing to lower rates of treatment failure or improve survival. While phase I/II data for novel treatment strategies are emerging, such as the incorporation of lenalidomide or ibrutinib into R-CHOP,[42-44] another approach worth reconsidering is consolidation RT.
Radiation Therapy
Radiation may be the most active single agent in the treatment of DLBCL.[3-6] Consolidation RT has demonstrated improved outcomes for patients with early-stage DLBCL.[11,12,45-47] In both early and advanced DLBCL treated with chemotherapy alone, the most common sites of treatment failure are those in which disease was initially involved,[48] particularly in patients with bulky lesions.[49,50] Consolidation RT after systemic chemotherapy has been shown to decrease the risk of relapse at treated sites and is often feasible if the disease is not too widely disseminated. There are at least five institutional experiences that support improved outcomes with consolidation RT for advanced-stage DLBCL.[10,49-52] Furthermore, post-hoc analysis from the RICOVER-60 trial and early results from the UNFOLDER randomized trial (Unfavorable Low-Risk Patients Treated With Densification of R-Chemo Regimens; ClinicalTrials.gov identifier NCT00278408) further support the role of consolidation RT.
Institutional experiences
Investigators at The University of Texas MD Anderson Cancer Center reviewed outcomes in patients with DLBCL treated with R-CHOP chemotherapy (Table).[10] The report included 469 patients with all stages of disease, but approximately 60% were stage III/IV and 37% had bulky disease (> 5 cm). Of 279 patients with stage III/IV disease, 39 (14%) were treated with consolidation RT. Reasons for RT utilizations were not given. Among complete responders to R-CHOP, in-field control was 100% with the addition of RT (local control data without RT were not provided), translating into improved OS (89% with RT added vs 66% with R-CHOP alone; P = .0008) and PFS (76% vs 55%; P = .003) for patients receiving consolidation RT. Matched-pair analysis, including patients with advanced disease, also showed that RT improved OS and PFS.
We have reported our experience at Duke utilizing consolidation RT in patients with advanced-stage DLBCL (see Table).[52] Our retrospective series included 79 patients, all with stage III/IV DLBCL, who achieved a CR to chemotherapy by functional imaging (FDG-PET [18-fluorine fluorodeoxyglucose positron emission tomography] or gallium scan). The majority (65%) were treated with R-CHOP. A total of 38 patients were treated with low-dose consolidation RT (at a median dose of 25 Gy), and 24 of these patients received RT to all sites of disease prior to chemotherapy. Consolidation RT was associated with improved in-field control (92% with R-CHOP plus RT vs 69% with R-CHOP; P = .028), PFS (85% vs 65%, respectively; P = .014), and a trend toward improved OS (85% vs 78%, respectively; P = .15). Multivariate analysis confirmed the benefit of RT for in-field control (hazard ratio [HR] = 8.01; P = .014) and PFS (HR = 4.3; P = .014). It should be noted that these benefits were seen despite use of lower-than-traditional doses of RT and the presence of larger tumors (median 7 cm vs 4 cm; P = .012) in patients who received consolidation RT.
A report from Emory University’s Winship Cancer Institute details patterns of failure among patients with advanced-stage DLBCL receiving R-CHOP chemotherapy, with or without consolidation RT (see Table).[51] Among a total of 211 patients presenting with stage III/IV disease, 110 (67.5%) achieved a CR by PET/CT after treatment with a median of 6 cycles of R-CHOP–like chemotherapy. Of 110 complete responders, 14 were treated with consolidation RT. Patients treated with RT had more bulky disease (≥ 5 cm), but otherwise their characteristics did not differ significantly from those of patients not receiving RT. Consolidation RT not only improved local control (91.7% vs 48.8% without RT) but also improved distant control (92.9% vs 71.9%, respectively), PFS (85.1% vs 44.2%), and OS (92.3% vs 68.5%; P < .0001 for all comparisons). On multivariate analysis, consolidation RT was associated with improved local control and PFS, and a trend toward improved OS (P = .08). Of 45 treatment failures in patients who did not receive RT, 42 included a component of local failure.
Two additional institutional studies evaluating the role of RT in advanced DLBCL predate the widespread adoption of functional imaging and rituximab[49,50] but nonetheless provide valuable proof of principle supporting the role of RT in advanced-stage DLBCL. The first, from the Scientific Institute Hospital San Raffaele, Milan, Italy, reviewed outcomes for patients with stage III/IV, bulky (≥ 10 cm), semi-bulky (6–9 cm), or extranodal disease who achieved a CR to induction chemotherapy.[49] Of 94 patients identified, 40 (20 with bulky disease and 20 with semi-bulky disease) were treated with consolidation RT (median dose, 38 Gy). Multivariate analysis controlling for IPI score confirmed the benefit of RT for EFS and OS. Similarly, a study from MD Anderson Cancer Center reported on outcomes for 59 patients enrolled on two prospective protocols, of whom 28 were treated with consolidation RT.[50] Radiation improved 5-year local control (89% for those treated with chemotherapy plus RT vs 52% for those treated with chemotherapy alone; P = .0001) and freedom from progression (85% vs 51%, respectively; P = .0003). The benefit was primarily due to the improvements for patients with tumors ≥ 4 cm, with 5-year local control rates of 89% with chemotherapy plus RT vs 33% with chemotherapy alone (P = .0009).
In sum, these retrospective experiences confirm that the most common sites of recurrence after chemotherapy alone are at initially involved sites. In most reports, it is neither clear what the selection criteria were for administering RT nor what fields were irradiated. In the Duke University series, the majority of patients received RT to all known sites of disease prior to chemotherapy. In each series, consolidation RT significantly improved local control, PFS, and often OS. The studies from MD Anderson, Emory, and Duke all show a benefit, even when a CR is achieved by PET. Further, the Duke experience suggests the benefits of consolidation RT can be realized with fairly low doses (18 to 24 Gy) of radiation.
Bulky disease
One clinical scenario that warrants special consideration of consolidation RT is the presence of bulky disease, such as a large mediastinal or abdominal mass, with or without widespread systemic involvement. RICOVER-60 was a four-arm study comparing four different chemotherapy regimens. Consolidation RT (36 Gy) was delivered to sites of initially bulky disease (≥ 7.5 cm) and/or extralymphatic involvement.[28] As an amendment to the original trial, an additional cohort of patients was accrued and treated with the most effective regimen from the randomized trial (R-CHOP-14 × 6 cycles) but without consolidation RT[53]; this study design was referred to as the RICOVER-noRTh trial. A total of 164 patients were enrolled and compared with outcomes in 111 patients who received R-CHOP-14 × 6 with consolidation RT during the original randomized trial. A multivariate analysis, controlling for IPI and age (> 70 years), found that among patients with bulky disease, the addition of consolidation RT improved PFS (HR = 4.4; P = .001) and OS (HR = 4.3; P = .002). Three-year PFS rates were 75% and 61% (P < .001) for those treated with and without RT, respectively. Corresponding rates of OS were 90% and 65% (P = .001).
An additional study supporting the use of consolidation RT comes from the ongoing UNFOLDER trial. This study randomizes patients with bulky or extranodal DLBCL to R-CHOP-14 or R-CHOP-21. In a 2 × 2 factorial design, a second randomization to consolidation RT or observation was planned for patients achieving a CR to induction therapy. The arms omitting RT were closed early when interim analysis found excessive failures were observed when RT was omitted.
Skeletal involvement
Another potential indication for consolidation RT includes patients with extranodal disease, specifically those with skeletal involvement. The DSHNHL retrospectively assessed data from nine prospective trials (including MInT and RICOVER-60) to determine the prognostic significance of, and optimal treatment for, skeletal involvement in patients with aggressive B-cell lymphomas.[54] Multivariate analysis among patients treated with rituximab-based induction therapy showed skeletal involvement portended worse EFS. However, EFS was improved with consolidation RT to sites of skeletal involvement, regardless of disease stage.
Partial response
Numerous retrospective studies have established that a positive post-chemotherapy PET scan is predictive of in-field relapse and worse long-term outcomes compared with a negative PET result.[55-60] Typically in this patient population, progression occurs in more than 50% of patients. Consolidation RT has been explored as an attempt to sterilize residual local disease and achieve long-term disease control. We have assessed our Duke experience of consolidation RT in patients with post-induction PET scans.[60] Outcomes of 99 patients treated with RT were assessed based on post-induction PET scan. Twenty-one patients with a positive PET scan were treated (median dose, 36 Gy), resulting in 4-year local control of 71% and a favorable 5-year PFS of 65%. Of the remaining 78 patients with a negative PET, consolidation RT (median dose, 30 Gy) led to 5-year local control of 92% and PFS of 83%. A separate series from Harvard reported outcomes for 61 patients treated with R-CHOP chemotherapy and consolidation RT (median dose, 36 Gy).[61] Of these, 20 patients had a positive interim or post-induction PET scan, yet only one in-field recurrence occurred after RT. Three-year PFS rates for patients with negative and positive PET scans were very favorable in both groups, at 97% and 90%, respectively.
A series from the British Columbia Cancer Agency provides yet further support for the use of RT in the setting of PET-positive disease following induction chemotherapy.[62] Per institutional policy, all patients with residual masses > 2 cm on CT underwent a PET scan, followed by consolidation RT to all PET-positive sites. Those with a negative PET were observed regardless of initial or residual bulk. Of 60 patients with a positive PET who received RT, only 6 relapsed in field, translating into a 4-year OS equivalent to that of the 167 patients who had a negative PET (85% vs 83%, respectively). Among 22 patients who had a positive PET but did not receive RT for various reasons, 4-year OS was 30%. In summary, while a positive interim or post-induction PET scan indicates a worse prognosis, consolidation RT can achieve local control and long-term outcomes similar to those observed in patients with negative PET scan results.
Radiation dose
Radiation doses utilized for patients with a variety of lymphoma histologies have been reduced over the last decade. A recent randomized study from the United Kingdom demonstrated no difference between RT at 30 Gy vs 45 Gy in patients with DLBCL.[63] For stage I/II disease, a dose of 30 Gy is currently considered standard therapy in the setting of a negative PET scan post chemotherapy. When delivering consolidation RT for treatment of advanced DLBCL, it is reasonable to consider lower doses than those used in localized disease. These patients will not be cured if systemic therapies are not effectively killing widespread subclinical disease. Low-dose consolidation RT may be all that is needed for controlling clinically evident disease in the setting of a CR following chemotherapy. Such patients are also typically treated with more cycles of chemotherapy and thus presumably have a lower burden of residual disease. Furthermore, treatment volumes may be larger in this patient population and reducing acute and long-term toxicity is important.
Our current approach is to use doses of 18–24 Gy in the setting of a CR to chemotherapy. Low doses such as these appear to be equally effective compared with larger doses[64] and are most likely associated with lower risks of acute and late toxicity. With such doses, local control in our experience has been 94%, consistent with series in which doses of 30–40 Gy have been utilized.[10,51,65] Our treatment volumes include all sites of original involvement when clinically feasible.
Higher doses are warranted in the setting of a positive post-chemotherapy PET scan. With chemotherapy alone, lack of a CR as determined by PET is associated with a substantial risk of relapse.[55,56,58,66,67] Even with combined modality therapy, a post-chemotherapy PET is associated with inferior outcomes.[66] In a study from Duke, 4-year rates of local control were 97% vs 81% (P < .01) for negative and positive post-chemotherapy PET scans, respectively. The corresponding median RT doses were 30 Gy and 36 Gy, respectively. In the setting of a partial response with positive post-chemotherapy PET scan, doses of 36–40 Gy are generally warranted.
Radiation fields
In all scenarios, we recommend adherence to involved-site RT as defined in the recently published guidelines from the International Lymphoma Radiation Oncology Group.[68] In general, this involves delineating the pre-
chemotherapy tumor volumes, excluding organs that were clearly uninvolved but which were displaced by the tumor. A planning volume is generated by adding an appropriate margin, accounting for patient setup variation and organ motion.
Conclusions and Case Examples
In conclusion, current treatment approaches rely heavily on systemic chemotherapy and immunotherapy. However, local recurrence remains a significant cause of treatment failure. RT represents an effective local therapy for DLBCL, and its incorporation into the treatment of advanced DLBCL needs to be considered. A growing body of retrospective and prospective data demonstrates the ability of RT to prevent local recurrences in patients with DLBCL, thereby improving both PFS and OS. To illustrate this point, a variety of clinical scenarios involving patients with advanced-stage DLBCL are presented below.
Stage IV disease of limited extent
A case example of this clinical scenario is depicted in Figure 1. This 77-year-old woman presented with mediastinal and bilateral hilar adenopathy, a right lower lobe nodule, and a single involved retroperitoneal lymph node (stage IVA). She received 6 cycles of R-CHOP, achieving a CR by PET. In addition, she was treated with low-dose (18 Gy) RT to all sites of disease, including the lung nodule. She is now disease-free 6 years after completing consolidation RT.
With an understanding that RT improves outcomes in stage I/II disease, incorporating RT in the treatment of patients with limited advanced disease is rational. The diaphragm is an arbitrary divider of stage I/II and stage III/IV presentations, and patients with limited disease on both sites of the diaphragm also likely benefit from consolidation RT. Due to the limited extent of disease in the case described here, treating all initial sites of disease was feasible and safe.
Stage IV DLBCL with bulky disease
A case example is depicted in Figure 2. This 61-year-old man presented with a large abdominal mass with extensive mesenteric nodal disease adjacent to the mass.
There were other involved sites, including the left iliac bone, the right inguinal region, T4 vertebral body, a small superior mediastinal lymph node, and the left supraclavicular fossa (stage IVA). He was treated with 6 cycles of R-CHOP and achieved a CR by PET. He went on receive low-dose (18 Gy) consolidation RT to the initially involved bulky abdominal disease and immediately adjacent mesenteric adenopathy. He is disease-free 3 years after completing RT.
While it was not feasible in this case to treat all sites of disease, the bulky disease was considered the most likely site of treatment failure. Radiation in this setting was delivered at a low dose to minimize toxicity.
Extranodal disease (skeletal involvement)
A case example is depicted in Figure 3. This 53-year-old man presented with a large mediastinal mass with pericardial lymphadenopathy. He also had involvement of the left iliac crest with B-symptoms at presentation (Stage IVB). The patient was treated with 6 cycles of R-CHOP and achieved a CR by PET imaging. He was treated with consolidation RT (20 Gy) to the mediastinum with a modified mantle field, as well as to the involved iliac crest, and is disease-free 7 years after RT. Treatment to all sites of disease, including the osseous site, was considered to be safe and feasible.
Stage IV DLBCL with partial response
A case example is depicted in Figure 4. This 51-year-old man presented with a large right chest wall mass with diffuse skeletal disease, pulmonary nodules, and cervical adenopathy. He was treated with 6 cycles of R-CHOP. Post-chemotherapy PET showed a CR at all sites except the chest wall mass (Deauville 4). He received consolidation RT (40 Gy) to the chest wall mass only. Post-RT PET showed interval improvement, and the patient is disease-free 1 year after RT. Because a CR was not achieved in the chest wall, this region was treated with a higher dose of radiation in an effort to establish local control and prevent treatment failure.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265-76.
2. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-95.
3. Kaminski MS, Coleman CN, Colby TV, et al. Factors predicting survival in adults with stage I and II large-cell lymphoma treated with primary radiation therapy. Ann Intern Med. 1986;104:747-56.
4. Monfardini S, Banfi A, Bonadonna G, et al. Improved five year survival after combined radiotherapy-chemotherapy for stage I-II non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 1980;6:125-34.
5. Nissen NI, Ersboll J, Hansen HS, et al. A randomized study of radiotherapy versus radiotherapy plus chemotherapy in stage I-II non-Hodgkin’s lymphomas. Cancer. 1983;52:1-7.
6. Vaughan Hudson B, Vaughan Hudson G, MacLennan KA, et al. Clinical stage 1 non-Hodgkin’s lymphoma: long-term follow-up of patients treated by the British National Lymphoma Investigation with radiotherapy alone as initial therapy. Br J Cancer. 1994;69:1088-93.
7. Landberg TG, Hakansson LG, Moller TR, et al. CVP-remission-maintenance in stage I or II non-Hodgkin’s lymphomas: preliminary results of a randomized study. Cancer. 1979;44:831-8.
8. Yahalom J, Varsos G, Fuks Z, et al. Adjuvant cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy after radiation therapy in stage I low-grade and intermediate-grade non-Hodgkin lymphoma. Results of a prospective randomized study. Cancer. 1993;71:2342-50.
9. Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26:2258-63.
10. Phan J, Mazloom A, Medeiros J, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28:4170-6.
11. Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032-8.
12. Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339:21-6.
13. Zelenetz AD, Wierda WG, Abramson JS, et al. Non-Hodgkin’s lymphomas, version 1.2013. J Natl Compr Canc Netw. 2013;11:257-72.
14. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987-94.
15. Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9:105-16.
16. Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817-26.
17. Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14:525-33.
18. Cooper IA, Wolf MM, Robertson TI, et al. Randomized comparison of MACOP-B with CHOP in patients with intermediate-grade non-Hodgkin’s lymphoma. The Australian and New Zealand Lymphoma Group. J Clin Oncol. 1994;12:769-78.
19. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002-6.
20. Gordon LI, Harrington D, Andersen J, et al. Comparison of a second-generation combination chemotherapeutic regimen (m-BACOD) with a standard regimen (CHOP) for advanced diffuse non-Hodgkin’s lymphoma. N Engl J Med. 1992;327:1342-9.
21. Reyes F, Lepage E, Ganem G, et al. ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005;352:1197-205.
22. Tilly H, Lepage E, Coiffier B, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102:4284-9.
23. Shipp MA, Harrington DP, Klatt MM, et al. Identification of major prognostic subgroups of patients with large-cell lymphoma treated with m-BACOD or M-BACOD. Ann Intern Med. 1986;104:757-65.
24. Longo DL, DeVita VT, Jr, Duffey PL, et al. Superiority of ProMACE-CytaBOM over ProMACE-MOPP in the treatment of advanced diffuse aggressive lymphoma: results of a prospective randomized trial. J Clin Oncol. 1991;9:25-38.
25. Klimo P, Connors JM. MACOP-B chemotherapy for the treatment of diffuse large-cell lymphoma. Ann Intern Med. 1985;102:596-602.
26. Greb A, Bohlius J, Schiefer D, et al. High-dose chemotherapy with autologous stem cell transplantation in the first line treatment of aggressive non-Hodgkin lymphoma (NHL) in adults. Cochrane Database Syst Rev. 2008;CD004024.
27. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma.
N Engl J Med. 2002;346:235-42.
28. Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013-22.
29. Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117-26.
30. Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040-5.
31. Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121-7.
32. Recher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378:1858-67.
33. Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104:634-41.
34. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma.
N Engl J Med. 1995;333:1540-5.
35. Freedman AS, Takvorian T, Neuberg D, et al. Autologous bone marrow transplantation in poor-prognosis intermediate-grade and high-grade B-cell non-Hodgkin’s lymphoma in first remission: a pilot study. J Clin Oncol. 1993;11:931-6.
36. Nademanee A, Molina A, O’Donnell MR, et al. Results of high-dose therapy and autologous bone marrow/stem cell transplantation during remission in poor-risk intermediate- and high-grade lymphoma: international index high and high-intermediate risk group. Blood. 1997;90:3844-52.
37. Vitolo U, Cortellazzo S, Liberati AM, et al. Intensified and high-dose chemotherapy with granulocyte colony-stimulating factor and autologous stem-cell transplantation support as first-line therapy in high-risk diffuse large-cell lymphoma. J Clin Oncol. 1997;15:491-8.
38. Martelli M, Vignetti M, Zinzani PL, et al. High-dose chemotherapy followed by autologous bone marrow transplantation versus dexamethasone, cisplatin, and cytarabine in aggressive non-Hodgkin’s lymphoma with partial response to front-line chemotherapy: a prospective randomized Italian multicenter study.
J Clin Oncol. 1996;14:534-42.
39. Kluin-Nelemans HC, Zagonel V, Anastasopoulou A, et al. Standard chemotherapy with or without high-dose chemotherapy for aggressive non-Hodgkin’s lymphoma: randomized phase III EORTC study. J Natl Cancer Inst. 2001;93:22-30.
40. Verdonck LF, van Putten WL, Hagenbeek A, et al. Comparison of CHOP chemotherapy with autologous bone marrow transplantation for slowly responding patients with aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1995;332:1045-51.
41. Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681-90.
42. Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-cell lymphoma: a phase II study. J Clin Oncol. 2014 Aug 18. [Epub ahead of print]
43. Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 2014;15:1019-26.
44. Vitolo U, Chiappella A, Franceschetti S, et al. Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol. 2014;15:730-7.
45. Martinelli G, Gigli F, Calabrese L, et al. Early stage gastric diffuse large B-cell lymphomas: results of a randomized trial comparing chemotherapy alone versus chemotherapy + involved field radiotherapy. (IELSG 4). [corrected]. Leuk Lymphoma. 2009;50:925-31.
46. Miller TP, LeBlanc M, Spier C. CHOP alone compared to CHOP plus radiotherapy for early stage aggressive non-Hodgkin’s lymphomas: update of the Southwest Oncology Group (SWOG) randomized trial. Blood. 2001;98:724a.
47. Ballonoff A, Rusthoven KE, Schwer A, et al. Outcomes and effect of radiotherapy in patients with stage I or II diffuse large B-cell lymphoma: a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys. 2008;72:1465-71.
48. Bonnet C, Fillet G, Mounier N, et al. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2007;25:787-92.
49. Ferreri AJ, Dell’Oro S, Reni M, et al. Consolidation radiotherapy to bulky or semibulky lesions in the management of stage III-IV diffuse large B cell lymphomas. Oncology. 2000;58:219-26.
50. Schlembach PJ, Wilder RB, Tucker SL, et al. Impact of involved field radiotherapy after CHOP-based chemotherapy on stage III-IV, intermediate grade and large-cell immunoblastic lymphomas. Int J Radiat Oncol Biol Phys. 2000;48:1107-10.
51. Shi Z, Das S, Okwan-Duodu D, et al. Patterns of failure in advanced stage diffuse large B-cell lymphoma patients after complete response to R-CHOP immunochemotherapy and the emerging role of consolidative radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:569-77.
52. Dorth JA, Prosnitz LR, Broadwater G, et al. Impact of consolidation radiation therapy in stage III-IV diffuse large B-cell lymphoma with negative post-chemotherapy radiologic imaging. Int J Radiat Oncol Biol Phys. 2012;84:762-7.
53. Held G, Murawski N, Ziepert M, et al. Role of radiotherapy to bulky disease in elderly patients with aggressive B-cell lymphoma. J Clin Oncol. 2014;32:1112-8.
54. Held G, Zeynalova S, Murawski N, et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol. 2013;31:4115-22.
55. Juweid ME, Cheson BD. Role of positron emission tomography in lymphoma. J Clin Oncol. 2005;23:4577-80.
56. Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571-8.
57. Juweid ME, Wiseman GA, Vose JM, et al. Response assessment of aggressive non-Hodgkin’s lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23:4652-61.
58. Mikhaeel NG, Hutchings M, Fields PA, et al. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514-23.
59. Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin’s lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414-9.
60. Dorth JA, Chino JP, Prosnitz LR, et al. The impact of radiation therapy in patients with diffuse large B-cell lymphoma with positive post-chemotherapy FDG-PET or gallium-67 scans. Ann Oncol. 2011;22:405-10.
61. Halasz LM, Jacene HA, Catalano PJ, et al. Combined modality treatment for PET-positive non-Hodgkin lymphoma: favorable outcomes of combined modality treatment for patients with non-Hodgkin lymphoma and positive interim or postchemotherapy FDG-PET. Int J Radiat Oncol Biol Phys. 2012;83:e647-54.
62. Sehn LH, Klasa R, Shenkier T, et al. Long-term experience with PET-guided consolidation radiation therapy (XRT) in patients with advanced stage diffuse large B-cell lymphoma (DLBCL) treated with R-CHOP. Hematological Oncology. 2013;31:96-150.
63. Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100:86-92.
64. Dorth JA, Prosnitz LR, Broadwater G, et al. Radiotherapy dose-response analysis for diffuse large B-cell lymphoma with a complete response to chemotherapy. Radiat Oncol. 2012;7:100.
65. Dorth JA, Broadwater G, Prosnitz LR, Kelsey CR. Impact of consolidative radiation therapy in stage III-IV diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2010;78(suppl):S552.
66. Dorth J, Chino J, Prosnitz L, Kelsey C. Positive PET prior to consolidative radiation therapy increases risk of in-field failure in diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2009;75(suppl):S64.
67. Mikhaeel NG, Timothy AR, O’Doherty MJ, et al. 18-FDG-PET as a prognostic indicator in the treatment of aggressive Non-Hodgkin’s Lymphoma-comparison with CT. Leuk Lymphoma. 2000;39:543-53.
68. Illidge T, Specht L, Yahalom J, et al. Modern radiation therapy for nodal non-Hodgkin lymphoma-target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2014;89:49-58.
Highlighting Insights From the Marginal Zone Lymphoma Workshop
Clinicians outline the significance of the MZL Workshop, where a gathering of international experts in the field discussed updates in the disease state.