Clinical Research of EGFR Inhibitors and Related Dermatologic Toxicities
An acneiform-like skin toxicity is commonly observed in patients with solid tumors treated with epidermal growth factor receptor inhibitors (EGFRIs). This symptomatic rash is related to epidermal growth factor receptor (EGFR) inhibition in the skin. A positive relation between the presence and severity of treatment-related rash and survival has been consistently observed with all EGFRIs approved for clinical use. These findings suggest that rash may be a useful surrogate marker of successful EGFR inhibition and clinical benefit and therefore of possible use in identifying patients most likely to benefit from therapy, as well as to guide dose adjustments. Increasing drug dose until skin toxicity appears is being studied. Further studies are needed to thoroughly evaluate the value of skin toxicity as a surrogate marker for clinical benefit. Current treatments of the skin toxicity are empirical and oriented toward mitigating symptoms and not validated by well-controlled clinical trials. Rational treatments based on the biological mechanisms of the skin toxicity must be developed and tested in well-controlled clinical trials.
An acneiform-like skin toxicity is commonly observed in patients with solid tumors treated with epidermal growth factor receptor inhibitors (EGFRIs). This symptomatic rash is related to epidermal growth factor receptor (EGFR) inhibition in the skin. A positive relation between the presence and severity of treatment-related rash and survival has been consistently observed with all EGFRIs approved for clinical use. These findings suggest that rash may be a useful surrogate marker of successful EGFR inhibition and clinical benefit and therefore of possible use in identifying patients most likely to benefit from therapy, as well as to guide dose adjustments. Increasing drug dose until skin toxicity appears is being studied. Further studies are needed to thoroughly evaluate the value of skin toxicity as a surrogate marker for clinical benefit. Current treatments of the skin toxicity are empirical and oriented toward mitigating symptoms and not validated by well-controlled clinical trials. Rational treatments based on the biological mechanisms of the skin toxicity must be developed and tested in well-controlled clinical trials.
The involvement of epidermal growth factor receptor (EGFR)–mediated signaling pathways in a variety of human solid tumors makes targeted inhibition of EGFR a rational approach to anticancer therapy. Results from preclinical and clinical studies suggest that EGFR inhibitors (EGFRIs) have inherent antitumor activity against a range of malignancies, as well as the ability to enhance the effects of conventional chemotherapy and radiotherapy.[1,2]
An acneiform-like skin rash is a common toxicity observed with both EGFR-targeted monoclonal antibodies and tyrosine kinase inhibitors (TKIs). The rash, which is most commonly seen above the waist and particularly around the nose, is characterized by clusters of monomorphic pustular lesions. The condition appears to be dose related, symptomatic to the point of requiring intervention in about one-third of patients, and self-limiting, usually resolving on discontinuation of therapy. The rash is thought to be directly related to inhibition of EGFR in the skin, leading to follicular occlusion as a consequence of the lack of differentiation in the epithelium, and release of cytokines that lead to epithelial inflammation.
The EGFR is expressed ubiquitously in epithelial and stromal cells, as well as in select glial and smooth-muscle cells.[3] In normal skin, the EGFR is constitutively expressed in the epidermis, sebaceous glands, and hair follicle epithelium.[4,5] Under physiologic conditions, EGFR activity is tightly regulated and mediates normal cellular growth and proliferation, such as the differentiation of skin follicles and keratinocytes.[6] Murine studies indicate that complete or partial abrogation of the EGFR gene causes significantly altered and thinned epidermis and abnormal colonic mucosa, which suggests that the downstream signaling cascade for EGFR is essential for normal development of epithelial tissues.[7,8]
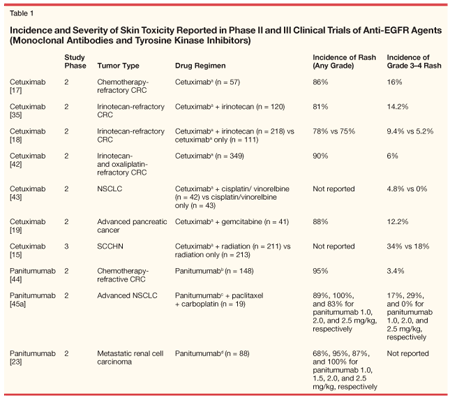
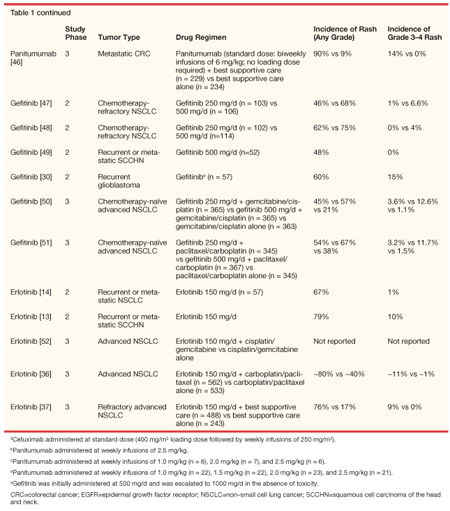
The acneiform-like skin toxicity characteristic of EGFR inhibition likely reflects the positive EGFR status of epidermal keratinocytes and other skin cells and supports the hypothesis that the antitumor effects of EGFRIs are caused by inhibition of EGFR signaling.[9] A consistently high incidence of rash, which generally takes the form of erythematous follicular papules and pustules, has been observed in patients treated with EGFR-specific monoclonal antibodies (eg, cetuximab [Erbitux], panitumumab [Vectibix], or matuzumab) and with small-molecule EGFR TKIs (eg, gefitinib [Iressa] and erlotinib [Tarceva], see Table 1).
Some anti-EGFR monoclonal antibodies, such as RG83852, do not inhibit the tyrosine kinase activity of the receptor, but are actually partially agonistic. In a phase I study of RG83852,[10] no skin rash was observed at doses that produced a high level of saturation of EGFR in vivo. This observation provides further support of the notion that the skin rash requires effective EGFR tyrosine kinase (TK) inhibition; however, TK inhibition may be necessary, but not sufficient, for the skin rash to occur.
A certain susceptibility to development of skin rash may be important because the degree of toxicity varies from patient to patient, and a significant number of patients do not develop rash at doses that result in significant EGFR TK inhibition. Studies to determine and characterize the possible immunologic basis of this individual susceptibility are needed to more fully understand the pathophysiology of the rash and to interpret the clinical observations that suggest a link between the occurrence of rash and improved survival. Rash may be an indicator of the degree of EGFR function inhibition and may predict objective tumor response or clinical benefit in cancer patients. The presence and severity of the rash could, therefore, serve as a useful guide for early selection of patients to continue treatment or for titrating EGFRI dosing to achieve an optimal response.
Introduction to EGFR Inhibitors
Monoclonal Antibodies
Cetuximab and panitumumab are the two anti-EGFR monoclonal antibodies currently approved by the US Food and Drug Administration (FDA) for use in cancer patients. Cetuximab, a chimeric monoclonal antibody, is approved in combination with irinotecan for the treatment of patients with irinotecan-refractory, EGFR-expressing metastatic colorectal cancer (mCRC) or as monotherapy for treatment of mCRC patients who are intolerant of irinotecan. In addition, cetuximab in combination with radiation therapy is approved for the treatment of patients with locally or regionally advanced squamous cell carcinoma of the head and neck (SCCHN), and single-agent cetuximab is approved for the treatment of patients with recurrent or metastatic SCCHN for whom previous platinum-based chemotherapy has failed (Erbitux package insert, 2006). Panitumumab, a fully human monoclonal antibody, is approved for treatment of patients with EGFR-expressing mCRC who have progressed on chemotherapy regimens containing fluoropyrimidine, oxaliplatin (Eloxatin), and irinotecan (Vectibix package insert, 2006).
Tyrosine Kinase Inhibitors
Gefitinib and erlotinib are the EGFR TKIs approved for cancer treatment. Gefitinib was initially approved by the FDA as monotherapy for third-line treatment of refractory advanced non–small-cell lung cancer (NSCLC) disease. However, in the Iressa Survival Evaluation in Lung Cancer (ISEL) trial, gefitinib did not improve survival or increase the time to treatment failure over placebo in the overall patient population or in patients with adenocarcinomas.[11]
Erlotinib demonstrated activity against NSCLC and other solid tumors in phase I and II trials.[12-14] The results of two phase III trials led to FDA approval of single-agent erlotinib as second-line therapy for locally advanced or metastatic NSCLC and of erlotinib in combination with gemcitabine as first-line therapy for locally advanced, unresectable, or metastatic pancreatic cancer (Tarceva package insert, 2006). Rash (59% to 79%) and diarrhea (37% to 86%) were the most common drug-related adverse events observed in clinical trials using doses of 100 to 200 mg daily.
Toxicities of EGFRIs
In phase II and III clinical trials of cetuximab at standard doses, alone and in combination with chemotherapy, the most frequently reported side effect was acneiform-like rash, occurring in 75% to 90% of cetuximab recipients (Table 1) (Erbitux package insert, 2006). Between 4.8% and 16% of patients receiving cetuximab developed rash of grade 3 or 4 severity as graded by the National Cancer Institute Common Toxicity Criteria (Erbitux package insert, 2006). Cetuximab in combination with radiation was associated with an incidence grade 3 or 4 skin reaction of 34% compared with 18% in patients receiving radiation alone (P = .0003).[15]
Cetuximab-induced skin rash is dose related.[16] The rash generally evolves within weeks 1 to 3 of treatment, with manifestations on the face, scalp, chest, and upper back.[16-20] Additional dermatologic manifestations include pain, tenderness, and fissuring of the distal finger tufts and paronychial inflammation associated with swelling or cracking of the lateral nail folds of toes and fingers.[17,19,20-22] Most affected patients experienced some degree of spontaneous partial improvement in the rash manifestation during the first 2 months of therapy.[16] Cessation of cetuximab generally results in complete reversal of the rash symptoms without scarring.[16]
Similar findings have been reported with panitumumab. More than 80% of patients receiving panitumumab at standard doses experienced skin rash (Table 1), generally manifesting in a periorificial manner on the face and upper trunk. As seen with cetuximab, the rash was generally asymptomatic and evident after 2 to 3 weeks of therapy, with maximal intensity between weeks 3 and 5.[23] Analysis of rash incidence in a phase II dose-escalating study suggests that the relation between dose and skin toxicity fits a sigmoidal curve, predicting a 90% incidence of skin rash from a 1.5-mg/kg dose of panitumumab (95% CI, 1.0–2.0 mg/kg).[23]
The acneiform-like rash in patients treated with the TKI erlotinib is similar to that reported with other EGFRIs (Table 1). In a phase I dose-finding trial, cutaneous toxicity was reported by 59% of patients. The rash generally reached maximum intensity at 2 weeks and resolved despite continuing treatment with erlotinib.[12] Rash occurred in 67% to 82% of patients with NSCLC, SCCHN, and ovarian cancer enrolled in phase II and III trials, appearing at a median of 7 to 10 days after treatment initiation.[13,24] The rash typically erupted around the mouth, nose, and upper torso and was characterized by clusters of monomorphic pustular lesions. Histopathologic evaluation revealed neutrophilic invasion of the dermal tissues, particularly the infundibular portion of the hair follicle, which is likely to have been caused by cytokine release by follicular cells or keratinocytes.[12] Although the cutaneous effects were visually prominent, the rash was generally asymptomatic or minimally symptomatic.[12] Immunohistochemical analysis revealed that, similar to other EGFR blockers, erlotinib increased expression of p27kip1 in skin tissue.[25] The degree of upregulation was dose related.
Clinical Implications of Skin Toxicity
Rash as a Surrogate Marker of Efficacy
Although EGFRIs represent a promising therapeutic strategy, identification of patients most likely to respond to therapy remains a clinical challenge, as does determining the most appropriate dosing level and schedule. Although the assumption that EGFR expression in tumors should correlate with clinical response to EGFRI therapies is supported by data in some studies,[26-28] benefit from treatment may not necessarily be predicted by EGFR protein-expression levels (measured by immunohistochemistry) or gene amplification (measured by fluorescence in situ hybridization) in pretreatment tumor samples.[24,29-31a-31c] However, in the cases of TKI inhibitors erlotinib and gefitinib for NSCLC, high gene copy number, mutations in the EGFR activation domain, and lack of K-ras mutations are emerging as potential molecular determinants of clinical benefit.[26,28,32-34a-34c] All of these findings must be validated prospectively by the use of validated assays.
The linear relation between EGFR inhibition and antitumor activity in in vitro settings suggests that EGFR degree of inhibition is a biologically relevant endpoint that could help to determine the appropriate dose. This approach differs from conventional chemotherapy, in which the maximum tolerated dose (usually pushed until bone marrow suppression is seen) is used to define the most appropriate dosing regimen. Most patients, however, do not have accessible tumors available for repetitive tissue biopsies, and given the unclear clinical relation between EGFR expression and objective tumor response or clinical benefit, other surrogate markers of therapeutic effect must be considered.
One potential marker of complete EGFR inhibition may be acneiform-like rash. Increasing the dosage until rash is observed ("dose-to-rash" strategy) has been shown to be feasible and to result in increased tumor response in the case of cetuximab but may be limited by gastrointestinal toxicity in the case of small-molecule TKIs.[55]
In patients treated with cetuximab or panitumumab, either as monotherapy or in combination with conventional chemotherapy, both the presence and intensity of acneiform rash predicts objective tumor response and survival. In patients with refractory colorectal cancer (CRC) receiving single-agent cetuximab, longer survival was observed in patients with rash of any grade compared with those with no rash (P = .02).[17] Furthermore, a trend toward increased survival was reported with increased rash severity grade.[17]
Similar results were found in a phase II trial in patients with refractory CRC receiving cetuximab alone or in combination with irinotecan.[18] Patients receiving cetuximab monotherapy with and without rash had median survival times of 8.1 and 2.5 months, respectively. Corresponding respective survival times in combination therapy patients were 9.1 and 3.0 months. A positive relationship between the presence and intensity of rash and survival was also demonstrated in patients with SCCHN receiving cetuximab in combination with cisplatin.[35]
These findings suggest that individualized dose titration, based on the appearance and severity of skin toxicity, may enable optimization of cetuximab therapy across a range of malignancies.
Similar results were observed in the phase II and phase III studies of panitumumab in patients with colorectal carcinoma.[43,45a-45b] In the phase II study, patients with grade 2-4 maximum skin toxicity had better progression-free survival ([HR], 0.67; 95% CI, 0.50-0.90) and overall survival (HR, 0.72; 95% CI, 0.54-0.97) compared with those with a maximum grade of 0-1.[43] This observation was further confirmed in the phase III study, in which PFS among patients in the panitumumab group also appeared to favor patients with a worst severity of grade 2-4 vs grade 1 skin toxicity (HR, 0.62; 95% CI, 0.44 to 0.88).[45a]
The presence of rash may also be a useful predictor of increased survival with erlotinib in patients with solid tumors. In phase II monotherapy studies in patients with NSCLC, SCCHN, and ovarian cancer, survival was longer in patients with rash than in patients without rash (P = .0001, P = .038. and P = .009, respectively).[24] The severity of rash was also predictive of survival. In patients with SCCHN, a statistically significant difference was seen in survival between patients with no rash and patients with grade 2 to 4 rash (P = .045); however, no statistically significant difference was seen in survival between patients with no rash and patients with grade 1 rash.[13]
In a phase II study of patients with NSCLC, median survival was 1.5 months in patients without rash, compared with 8.5 months in patients with grade 1 rash and 19.6 months in patients with grade 2 or 3 rash (all pairwise comparisons were statistically significant).[14] Similarly, an analysis of a large phase III study in NSCLC found that rash was associated with survival: median survival was 8.4 months in patients without rash compared with 10.8 months in patients with grade 1 rash, 13.5 months in patients with grade 2 rash, and 13.2 months in patients with grade 3 or 4 rash.[36]
In a study of erlotinib and gemcitabine in patients with pancreatic cancer, median survival at 1 year was significantly greater for patients with grade 2 rash (43%) than for patients with grade 1 rash (11%).[56] A relationship between rash and clinical benefit or survival was also observed in a large randomized study in patients with recurrent NSCLC receiving erlotinib vs placebo.[37] However, this study also showed a trend toward improved survival in patients in the placebo group who presented signs of skin inflammation related to other causes, which suggests the possibility that the occurrence of rash may be a prognostic indicator as it relates to the immunocompetence of the host.
Conclusion
In all studies with monoclonal antibodies and TKIs (monotherapy and in combination) where the relation between rash and survival was retrospectively analyzed and reported, the skin toxicity (incidence and/or grade) was found to be correlated with survival. In addition, patients who did not develop rash had a worse outcome than the patients in the control arm. The implications are that skin toxicity is probably a reflection of both effective dosing and target inhibition, as well as baseline immunocompetence.
Future Research Directions
Dose-to-Rash Studies
On the basis of the observations summarized above, patients with no or mild skin toxicity from EGFRIs could reasonably be hypothesized to benefit from dose escalation. Ongoing dose-to-rash clinical studies of cetuximab as monotherapy or in combination with irinotecan (EVEREST) and of erlotinib, as a single agent or in combination with cisplatin/docetaxel, are evaluating this potential (www.clinicaltrials.gov). In the EVEREST study, patients with EGFR-expressing irinotecan refractory metastatic colorectal cancer were randomized at day 22 if no rash or slight rash (grade 1) and no other toxicities occurred between the combination of the standard dose of cetuximab (250 mg/m2/wk) plus irinotecan and a gradually increasing dose of cetuximab (increase of 50 mg/m2 every 2 weeks until a maximum of 500 mg/m2/wk). It has been shown in this study that a dose of 500 mg/m2 of cetuximab is well tolerated by patients who had no or mild skin reactions to standard doses. Patients in the dose escalation had slightly more skin toxicity and diarrhea compared to patients treated with the standard dose, but the safety was certainly manageable.
Dose escalation resulted in a higher response rate (16% vs 30%). There was a trend toward a slightly longer progression-free survival in the dose escalation arm (3.9 vs 4.8 months with overlapping confidence intervals).[38,39]. In this study, sequential skin and tumor biopsies were taken and showed no relevant on-treatment differences in biomarkers (in skin) between baseline and post-randomization scores in the standard dose and dose escalation group. Gene expression analysis of baseline tumor samples identified molecular profiles that seem to be associated with outcome: eg, epiregulin and amphiregulin. The concentration of many plasma proteins varied from baseline to prerandomization and from baseline to post-treatment timepoints on-treatment concentration changes correlated with tumor response for several of these proteins.[38]
Dose-to-rash studies are also being performed with erlotinib. Doses up to 300 mg seem to be tolerated by patients who do not develop skin toxicity at 150 mg (Tarceva package insert, 2006). In addition, a study of high-dose vs regular-dose erlotinib is being performed in active smokers as active smokers have a lower systemic exposure to erlotinib because of accelerated liver metabolism. If all these studies show a correlation between dose and response rate, definitive studies using progression-free survival as an endpoint will be justified.
Immunologic Studies
The pathophysiology of the inflammatory reaction triggered by EGFR inhibition in the keratinocytes of the basal epidermal layers remains to be elucidated. Such elucidation should help develop or select anti-inflammatory agents that address the specific mechanisms of inflammation involved. These therapies should be more effective than the current empirical therapies used for the control of the symptoms related to EGFRI-induced skin toxicity. Once the pathophysiology of the rash is better understood, evaluation of the baseline immune status of the patients may help us also better understand why some patients do not develop skin toxicity. Dose intensity may not explain the lack of skin toxicity in all cases. A lack of susceptibility, which is a reflection of immunologic deficits, may be associated with a worse prognosis. That outcome would explain the dismal prognosis of patients who do not develop skin toxicity.
Development of New Management Strategies
All skin rash management schemes currently used are empirical and focused on symptom control.[53,54] New therapies based on the pathophysiology of the skin rash must be developed. These therapies include strategies aimed at interfering topically with the receptor inhibitory mechanisms of the anti-EGFR agents or at inhibiting specific mechanisms of the early inflammatory response that occurs as a result of EGFR inhibition in the skin.
One such strategy consists of the development of phosphatase inhibitors to prevent the effect of the anti-EGFR agents at the level of the receptor. Because the baseline activation of EGFR is greatly dependent on the activity of the specific phosphatase, inhibition of such phosphatase should result in persistent EGFR activation independently of the presence of anti-EGFR agents blocking the extracellular ligand-binding domain or specifically inhibiting the intracellular TK function. Such effects have been demonstrated in vitro with menadione, a vitamin K analog that inhibits different phosphatases through intracellular ROS generation. A topical formulation of menadione is being developed, and clinical trials will begin in the next few months.[40,41]
Summary
Strong evidence suggests that the skin toxicity secondary to EGFRIs should be viewed not only as a toxicity triggered by EGFR inhibition in the skin but also as a possible marker of clinical efficacy. This relationship should be evaluated more thoroughly to determine how to use it to improve the clinical benefit provided by these agents. Rational prevention and treatment strategies for the skin toxicity must be developed and tested in well-controlled clinical trials.
Disclosures:
Roman Perez-Soler has served as a consultant for Genentech, OSI Pharmaceuticals, Amgen, ImClone, and GlaxoSmithKline; has stock in Genentech, OSI Pharmaceuticals, Amgen, Novartis, and AstraZeneca; has received honoraria from Genentech, Amgen, OSI Pharmaceuticals, ImClone, AstraZeneca; and has received research funds from OSI Pharmaceuticals; Eric Van Cutsem has served as a consultant and received research funds from Roche.
Funding for this supplement was provided by Amgen.
References:
1. Baselga J, Arteaga CL: Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol 23:2445-2459, 2005.
2. Khalil MY, Grandis JR, Shin DM: Targeting epidermal growth factor receptor: Novel therapeutics in the management of cancer. Expert Rev Anticancer Ther 3:367-380, 2003.
3. Wells A: EGF receptor. Int J Biochem Cell Biol 31:637-643, 1999.
4. Green MR, Couchman JR: Distribution of epidermal growth factor receptors in rat tissues during embryonic skin development, hair formation, and the adult hair growth cycle. J Invest Dermatol 83:118-123, 1984.
5. Busam KJ, Tan LK, Granter SR, et al: Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol 12:786-793, 1999.
6. Jost M, Kari C, Rodeck U: The EGF receptor-An essential regulator of multiple epidermal functions. Eur J Dermatol 10:505-510, 2000.
7. Threadgill DW, Dlugosz AA, Hansen LA, et al: Targeted disruption of mouse EGF receptor: Effect of genetic background on mutant phenotype. Science 269:230-234, 1995.
8. Sibilia M, Wagner EF: Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269:234-238, 1995.
9. Herbst RS, Maddox AM, Rothenberg ML, et al: Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non–small-cell lung cancer and other solid tumors: Results of a phase I trial. J Clin Oncol 20:3815-3825, 2002.
10. Perez-Soler R, Donato NJ, Shin DM, et al: Tumor epidermal growth factor receptor studies in patients with non–small-cell lung cancer or head and neck cancer treated with monoclonal antibody RG 83852. J Clin Oncol 12:730-739, 1994.
11. Thatcher N, Chang A, Parikh P, et al: Gefitinib plus best supportive care in previously treated patients with refractory advanced non–small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366:1527-1537, 2005.
12. Hidalgo M, Siu LL, Nemunaitis J, et al: Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 19:3267-3279, 2001.
13. Soulieres D, Senzer NN, Vokes EE, et al: Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 22:77-85, 2004.
14. Perez-Soler R, Chachoua A, Hammond LA, et al: Determinants of tumor response and survival with erlotinib in patients with non–small-cell lung cancer. J Clin Oncol 22:3238-3247,2004.
15. Bonner JA, Giralt J, Harari PM, et al: Cetuximab prolongs survival in patients with locoregionally advanced squamous cell carcinoma of head and neck: A phase III study of high dose radiation therapy with or without cetuximab (abstract 5507). Proc Am Soc Clin Oncol 23:2004.
16. Cohen RB, Falcey JW, Paulter VJ, et al: Safety profile of the monoclonal antibody (MoAb) IMC-C225, an anti-epidermal growth factor receptor (EGFr) used in the treatment of EGFr positive tumors (abstract 1862). Proc Am Soc Clin Oncol 19:474a, 2000.
17. Saltz LB, Meropol NJ, Loehrer PJ Sr, et al: Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201-1208, 2004.
18. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
19. Xiong HQ, Rosenberg A, LoBuglio A, et al: Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II trial. J Clin Oncol 22:2610-2616, 2004.
20. Busam KJ, Capodieci P, Motzer R, et al: Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol 144:1169-1176, 2001.
21. Boucher KW, Davidson K, Mirakhur B, et al: Paronychia induced by cetuximab, an antiepidermal growth factor receptor antibody. J Am Acad Dermatol 47:632-633, 2002.
22. Monti M, Mancini LL, Ferrari B, et al: Complications of therapy and a diagnostic dilemma case. Case 2: Cutaneous toxicity induced by cetuximab. J Clin Oncol 21:4651-4653, 2003.
23. Rowinsky EK, Schwartz GH, Gollob JA, et al: Safety, pharmacokinetics, and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol 22:3003-3015, 2004.
24. Clark GM, Perez-Soler R, Siu L, et al: Rash severity is predictive of increased survival with erlotinib HCI (abstract 786). Proc Am Soc Clin Oncol 22:196, 2003.
25. Malik SN, Siu LL, Rowinsky EK, et al: Pharmacodynamic evaluation of the epidermal growth factor receptor inhibitor OSI-774 in human epidermis of cancer patients. Clin Cancer Res 9:2478-2486, 2003.
26. Cappuzzo F, Hirsch FR, Rossi E, et al: Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non–small-cell lung cancer. J Natl Cancer Inst 97:643-655, 2005.
27. Hirsch F, McCoy J, Cappuzzo F, et al: FISH and immunohistochemistry (IHC) can be used to select NSCLC patients who will not benefit from gefitinib treatment (abstract O-107). Lung Cancer 49:S38, 2005.
28. Tsao MS, Sakurada A, Cutz JC, et al: Erlotinib in lung cancer-Molecular and clinical predictors of outcome. N Engl J Med 353:133-144, 2005.
29. Chung KY, Shia J, Kemeny NE, et al: Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 23:1803-1810, 2005.
30. Rich JN, Reardon DA, Peery T, et al: Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol 22:133-142, 2004.
31a. Bailey LR, Kris M, Wolf M, et al: Tumor EGFR membrane staining is not clinically relevant for predicting response in patients receiving gefitinib ('Iressa', ZD1839) monotherapy for pretreated advanced non–small-cell lung cancer: IDEAL 1 and 2 (abstract LB-170). Proc Am Assoc Cancer Res 44:1362, 2003.
31b. Berlin J, Neubauer M, Swanson P, et al: Panitumumab antitumor activity in patients (pts) with metastatic colorectal cancer (mCRC) expressing > 10% epidermal growth factor receptor (EGFr). J Clin Oncol 24(suppl 18):3548 (abstract 3548), 2006.
31c. Hecht JR, Mitchell E, Baranda J, et al: Panitumumab activity in metastatic colorectal cancer (mCRC) patients (pts) with low or negative tumor epidermal growth factor receptor (EGFr) levels: An updated analysis. J Clin Oncol 24(suppl 18)3547a (abstract 3547), 2007.
32. Eberhard DA, Johnson BE, Amler LC, et al: Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non–small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 23:5900-5909, 2005.
33. Niho S, Kubota K, Goto K, et al: First-line single agent of gefitinib in patients (pts) with advanced non–small cell lung cancer (NSCLC): A phase II study (abstract 7059). Proc Am Soc Clin Oncol 23:628, 2004 (slide presentation available at www.asco.org).
34a. Bell DW, Lynch TJ, Haserlat SM, et al: Epidermal growth factor receptor mutations and gene amplification in non–small-cell lung cancer: Molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol 23:8081-8092, 2005.
34b. Juan T, Freeman D, Sarosi I, et al: Association of Somatic KRAS Gene Mutations and Clinical Outcome from a Phase 2 mCRC Trial of Panitumumab. Presented at the 9th World Congress of Gastrointestinal Cancer, June 20-27, 2007, Barcelona, Spain. Abstract 594.
34c. Amado RG, Wolf M, Peeters M, et al: Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer: Results from a randomized, controlled trial. Presented at the 14th Biannual European Cancer Conference (ECCO); Sep 23-27, 2007, Barcolona, Spain. Abstract 0007.
35. Saltz L, Kies M, Abbruzzese JL, et al: The presence and intensity of the cetuximab-induced acne-like rash predicts increased survival in studies across multiple malignancies (abstract 817). Proc Am Soc Clin Oncol 22:204, 2003.
36. Herbst RS, Prager D, Hermann R, et al: TRIBUTE-A phase III trial of erlotinib HC1 (OSI-774) combined with carboplatin and paclitaxel (CP) chemotherapy in advanced non–small cell lung cancer (NSCLC) (abstract 7011). Proc Am Soc Clin Oncol 23:617, 2004 (slide presentation available online at www.asco.org).
37. Shepherd FA, Pereira J, Ciuleanu TE, et al: A randomized placebo-controlled trial of erlotinib in patients with advanced non–small cell lung cancer (NSCLC) following failure of 1st line or 2nd line chemotherapy. A National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) trial (abstract 7022). Proc Am Soc Clin Oncol 23:2004 (slide presentation available online at: www.asco.org).
38. Van Cutsem E, Peeters M, Gelderblom H, et al: Cetuximab dose-escalation in mCRC patients with no or slight skin reactions on standard treatment (EVEREST) (abstract O-0034). Presented at the World Congress on Gastrointestinal Cancer, Barcelona, 2007.
39. Tejpar S, Peeters M, Humblet H, et al: Phase I/II study of cetuximab dose-escalation in patients with metastatic colorectal cancer (mCRC) with no or slight skin reactions on cetuximab standard dose treatment (EVEREST): Pharmacokinetic (PK), pharmacodynamic (PD) and efficacy data (abstract 4037). J Clin Oncol 25(suppl 18S):172s, 2007 (slide presentation available at www.asco.org).
40. Perez-Soler R, Zou Y, Li T, et al: Topical vitamin K3 (Menadione) prevents erlotinib and cetuximab-induced EGFR inhibition in the skin (abstract 3036). J Clin Oncol 24(suppl 18S):129s, 2006 (slide presentation available at www.asco.org).
41. Perez-Soler R, Zou Y, Li T, et al: Steroids and immunosuppressive agents potentiate the cytotoxicity of the EGFR inhibitor erlotinib (E) in human skin keratinocytes whereas Vit K3 exerts a protective effect: implications for the management of the skin rash (abstract 9124). J Clin Oncol 25(suppl 18S):523s, 2007 (slide presentation available at www.asco.org).
42. Lenz HJ, Van Cutsem E, Khambata-Ford S: Multicenter Phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin and fluoropyrimidines. J Clin Oncol 24: 4914-4921, 2006.
43. Rosell R, Daniel C, Ramlau R, et al: Randomized phase II study of cetuximab in combination with cisplatin (C) and vinorelbine (V) vs. CV alone in the first-line treatment of patients (pts) with epidermal growth factor receptor (EGFR)-expressing advanced non–small-cell lung cancer (NSCLC) (abstract 7012). Proc Am Soc Clin Oncol 23:618, 2004 (slide presentation available at www.asco.org).
44. Hecht JR, Patnaik A, Berlin J, et al: Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer 110(5):980-988, 2007.
45a. Crawford J, Sandler AB, Hammond LA, et al: ABX-EGF in combination with paclitaxel and carboplatin for advanced non-small cell lung cancer (NSCLC) (abstract 7083). Proc Am Soc Clin Oncol 23:634, 2004.
45b. VanCutsem E, Siena S, Humblet Y, et al: An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Annals of Oncology, in press.
46. Van Cutsem E, Peeters M, Siena S, et al: Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658-1664, 2007.
47. Fukuoka M, Yano S, Giaccone G, et al: Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non–small-cell lung cancer. J Clin Oncol 21:2237-2246, 2003.
48. Kris MG, Natale RB, Herbst RS, et al: Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non–small cell lung cancer: a randomized trial. JAMA 290:2149-2158, 2003.
49. Cohen EEW, Rosen F, Stadler WM, et al: Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 21:1980-1987, 2003.
50. Giaccone G, Herbst RS, Manegold C, et al: Gefitinib in combination with gemcitabine and cisplatin in advanced non–small-cell lung cancer: A phase III trial-INTACT 1. J Clin Oncol 22:777-784, 2004.
51. Herbst RS, Giaccone G, Schiller JH, et al: Gefitinib in combination with paclitaxel and carboplatin in advanced non–small-cell lung cancer: A phase III trial-INTACT 2. J Clin Oncol 22:785-794, 2004.
52. Gatzemeier U, Pluzanska A, Szczesna A, et al: Results of a phase III trial of erlotinib (OSI-774) combined with cisplatin and gemcitabine (GC) chemotherapy in advanced non-small cell lung cancer (NSCLC) (abstract 7010). Proc Am Soc Clin Oncol 23:617, 2004.
53. Segaert S, Van Cutsem E: Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 16:1425-1433, 2005.
54. Van Cutsem E: Challenges in the use of epidermal growth factor receptor inhibitors in colorectal cancer. The Oncologist 11:1010-1017, 2006.
55. Perez-Soler R: Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer 8 (suppl 1):S7-S14, 2006.
56. Wacker B, Nagrani T, Weinberg J, et al: Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 13:3913-3921, 2007.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.