Clinical Management of EGFRI Dermatologic Toxicities: The Japanese Perspective
Epidermal growth factor receptor inhibitors (EGFRIs) have demonstrated clinical activity in patients with non–small-cell lung cancer, pancreatic cancer, and colorectal cancer. EGFRIs are generally well tolerated, but reversible dermatologic toxicities are commonly associated with their use. Limited clinical evidence has characterized these adverse reactions as a class effect. For panitumumab (Vectibix), mild-to-moderate dermatologic toxicities are the most common associated adverse reactions. This report details the Japanese experience in the management of dermatologic toxicities associated with panitumumab use. Treatment selection for skin toxicity in Japan is also detailed, with a flowchart depicting strategies to treat various stages of dermatologic toxicities. Panitumumab was well tolerated in Japanese patients with advanced solid tumors, with a safety profile similar to that seen in non-Japanese patients.
Epidermal growth factor receptor inhibitors (EGFRIs) have demonstrated clinical activity in patients with non–small-cell lung cancer, pancreatic cancer, and colorectal cancer. EGFRIs are generally well tolerated, but reversible dermatologic toxicities are commonly associated with their use. Limited clinical evidence has characterized these adverse reactions as a class effect. For panitumumab (Vectibix), mild-to-moderate dermatologic toxicities are the most common associated adverse reactions. This report details the Japanese experience in the management of dermatologic toxicities associated with panitumumab use. Treatment selection for skin toxicity in Japan is also detailed, with a flowchart depicting strategies to treat various stages of dermatologic toxicities. Panitumumab was well tolerated in Japanese patients with advanced solid tumors, with a safety profile similar to that seen in non-Japanese patients.
In a study reported by Yamada et al,[1] investigators evaluated the safety and pharmacokinetics of panitumumab (Vectibix) monotherapy in Japanese patients with advanced solid tumors. Primary objectives were to assess safety and evaluate pharmacokinetics. The secondary objectives were to assess immunogenicity (antipanitumumab antibody response) and clinical efficacy (objective tumor response) in Japanese patients with advanced solid tumors.
Patients were enrolled sequentially into one of three cohorts-2.5 mg/kg weekly, 6.0 mg/kg every 2 weeks, and 9.0 mg/kg every 3 weeks-and continued treatment until disease progression or drug intolerability. Patients were enrolled into the next dose cohort following evaluation of dose-limiting toxicity and safety data 4 weeks after the first infusion on the previous cohort.
Dermatologic Toxicities Observed in This Study
A total of 18 patients (6 per cohort) were enrolled and received at least one dose of panitumumab. At the time of the report, the median follow-up time was 16.5 weeks in cohort 1, 13.5 weeks in cohort 2, and 12 weeks in cohort 3. No dose-limiting toxicities were reported in this study. Some degree of skin-related toxicity was seen in all patients. Dermatologic toxicity was defined as any toxicity in the integument or eye.
The dermatologic toxicities observed in this study were predictable and generally tolerable. The reaction often took the form of clusters of monomorphous pustular lesions that resembled an acneiform-type drug eruption. The manifestations were similar in most affected patients and typically involved the upper trunk and face. The dermatologic manifestations were usually associated with only minimal symptoms but were cosmetically distressing for some patients. The reactions typically occurred 1 week after administration of panitumumab and rarely affected patients as early as day 3. Treatment for these adverse effects continued for as many as 91 days, while patients were still on therapy.
Time to First Skin Toxicity
The first dermatologic toxicity was seen in a median of 7 days (Kaplan-Meier estimate; 95% confidence interval = 5–8 days). In cohort 1, the median was 7 days (range, 3–11); in cohort 2, it was 8 days (range, 5–9); and in cohort 3, it was also 8 days (range, 3–8). The median time to the most severe skin toxicity was day 11 (range, 8–14) in all patients (n = 18). In cohort 1, the median time to the most severe skin toxicity was 14 days (range, 7–20); in cohort 2, it was 11 days (range, 9–14); and in cohort 3, it was 9 days (range, 8–14) [Amgen data on file].
Treatment Selection for Skin Toxicity in Japan
EGFR inhibitors are generally well tolerated and do not have any of the severe systemic side effects seen with cytotoxic agents. However, these agents do cause skin toxicity, most often a papulopustular reaction (PPR).[2-5]
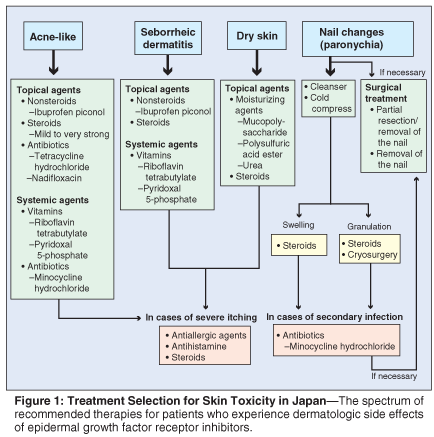
Figure 1 is a flowchart of treatment selection for skin toxicity in Japan (author recommendations). Mild cases of acneiform eruption respond well to topical anti-inflammatory therapy for acne. However, because some topical treatments have drying properties and may aggravate xerosis, the coadministration of emollients to address xerosis can be helpful. Ibuprofen piconol is typically prescribed at the onset of symptoms.
Infected rash may occur, and empiric use of an oral antibiotic is selected in some cases. When the presence of infection is suspected, antibiotics such as tetracycline hydrochloride or nadifloxacin are prescribed, as well as the bactericidal chlorhexidine gluconate. Other topical agents used include steroids (betamethasone butyrate propionate, clobetasol propionate, diflorasone diacetate), an anti-inflammatory agent (mucopolysaccharide polysulfuric acid ester), and urea. Similarly, systemic agents include both antibiotics (minocycline hydrochloride) and anti-inflammatory drugs (loxoprofen). Also used systemically are the antioxidant riboflavin tetrabutylate, and the active ingredient of vitamin B6, pyridoxal 5-phosphate.
The treatment selection flowchart described in this section is intended to show practice parameters in Japan. It is not intended to be used as a clinical practice guideline for optimal treatment of EGFRI-associated dermatologic toxicities, as there are inherent regional differences in practice patterns.
When to Refer to a Dermatologist
Dermatologic manifestations are best managed in close collaboration with oncologists and dermatologists who establish treatment strategies prior to the manifestation of toxicities. The predictable development of these symptoms within 7 to 9 days after initiating panitumumab therapy provides an opportunity to prepare patients for them. Although rashes, dry skin, and nail and hair reactions are rarely severe, they can cause discomfort and can be cosmetically distressing. The concern that some patients may request treatment interruption because of dermatologic toxicities should be addressed well before they appear.
For grade 1 reactions, these effects can be managed without referral to a dermatologist. As the severity of lesions advances to grade 2, closer consultation is required. All grade 3 or 4 effects should be managed collaboratively by an oncologist and a dermatologist.
Conclusions
Reversible dermatologic toxicities are common with EGFRI agents. The observed dermatologic toxicities were low-grade in this report from a study of Japanese patients. Acne-like lesions, exfoliative rash, pruritus, dry skin, paronychia, and skin fissures are the prominent manifestations of dermatologic toxicity associated with EGFRIs, but other adverse effects can be seen. In this report, panitumumab was well tolerated in Japanese patients, with limited grade 3 or 4 dermatologic toxicities reported.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Funding for this supplement was provided by Amgen.
References:
1. Yamada Y, Tamura T, Shirao K, et al: Safety and pharmacokinetics (PK) of panitumumab in Japanese patients (pts) with advanced solid malignancies (abstract 385). Presented at the 2007 Gastrointestinal Cancers Symposium. Available at www.asco.org. Accessed September 5, 2007.
2. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
3. Foon K, Yang X-D, Weiner L, et al: Preclinical and clinical evaluation of ABX-EGF, a fully human anti-EGFR antibody. Int J Radiat Oncol Biol Phys 58:984-990, 2004.
4. Kris M, Natale R, Herbst R, et al: Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer. JAMA 290:2149-2158, 2003.
5. Hidalgo M, Siu L, Nemunaitis J, et al: Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 19:3267-3279, 2001.