Collision Renal Cell Papillary and Medullary Carcinoma in a 66-Year-Old Man
The patient is a 66-year-old male who presented to his primary care physician with a 3-week history of painless gross hematuria. He underwent a renal ultrasound that showed a left kidney mass.
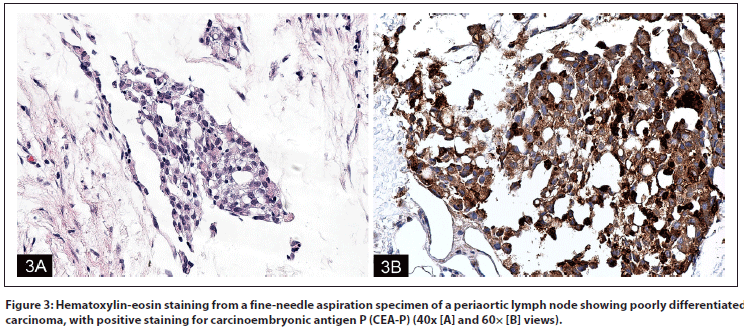
Figure 1: Nephrectomy specimen showing (A) poorly differentiated medullary carcinoma (large arrow) colliding with low-grade papillary carcinoma (small arrow) (10×); (B) higher-power views (40×) of low-grade papillary carcinoma (left, 1B-l) and medullary carcinoma (right, 1B-r); and (C) medullary carcinoma invasive into perirenal fat (10×); all images with hematoxylin-eosin staining.
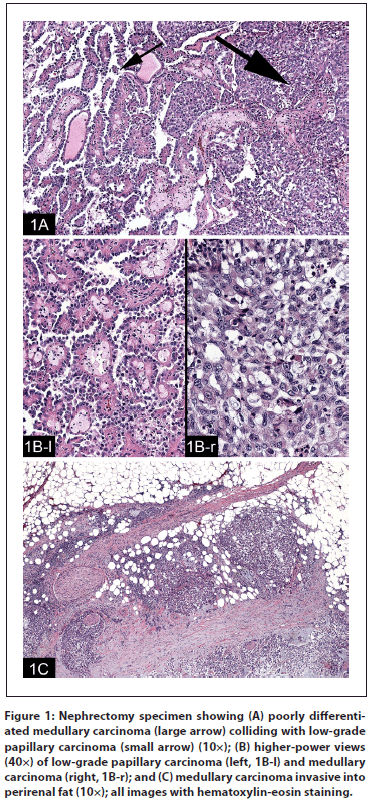
Figure 2: Nephrectomy specimen showing (A) staining for carcinoembryonic antigen P (CEAP), strongly positive in the poorly differentiated medullary tumor and negative in the papillary tumor; (B) staining for RCC, strongly positive in the papillary tumor and negative in the poorly differentiated medullary tumor; and (C) staining for racemase, strongly positive in the papillary tumor and negative in the poorly differentiated medullary tumor.
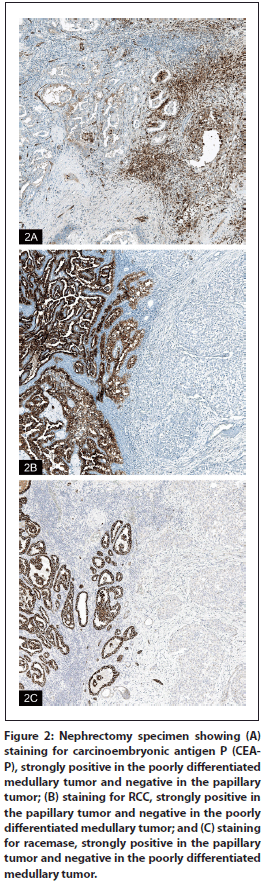
Figure 3: Hematoxylin-eosin staining from a fine-needle aspiration specimen of a periaortic lymph node showing poorly differentiated carcinoma, with positive staining for carcinoembryonic antigen P (CEA-P) (40x [A] and 60× [B] views).
The Case: The patient is a 66-year-old male who presented to his primary care physician with a 3-week history of painless gross hematuria. He underwent a renal ultrasound that showed a left kidney mass. A computed tomography (CT) scan of the abdomen and pelvis confirmed the presence of a left mid-anterior renal mass measuring 5.3 × 5.9 × 7.4 cm, with thrombus extension into a branch of the left renal vein coursing through the mass, but no extension into the main left renal vein. Excretory phase images showed extrinsic compression of the left intrarenal collecting system at the level of the mass. A previous CT scan of the pelvis, done 6 months earlier to evaluate flank pain, showed only slight lobulation in this area. A staging chest x-ray was unremarkable. The patient is a lifelong nonsmoker and has no personal or family history of malignancy, hematologic disorders, or sickle cell hemoglobinopathy. He underwent left radical nephrectomy with dissection of the renal vein. An outside pathology report described a unifocal, 7-cm, pT3aNx, Fuhrman grade 3-4/4 renal cell carcinoma (RCC) with rhabdoid features juxtaposed to papillary RCC. There was macroscopic extension into the renal vein, renal pelvis, and perinephric fat, and microscopic extension into the renal pelvis and perinephric fat. Postoperative laboratory evaluations were pertinent for a white blood cell count of 7.5 × 103/µL, absolute neutrophil count of 5.4 × 103/µL, hemoglobin level of 14.1 g/dL, platelets of 224 × 103/µL, creatinine level of 1.2 mg/dL, and calcium level of 9.1 mg/dL. Lactate dehydrogenase level was not available.
The patient participated in the Southwest Oncology Group S0931 phase III adjuvant trial in which patients were randomly assigned to treatment with either everolimus or placebo in the adjuvant setting. Pretreatment, baseline CT scans showed no evidence of metastatic disease. Restaging scans after 3 months on treatment showed interval development of multiple periaortic lymph nodes, which were more than 1 cm in longest diameter. He was unblinded after progression on study and was found to have been randomized to the placebo arm. A left periaortic lymph node was biopsied and found to be consistent with metastatic renal cell cancer. He was then treated with pazopanib and had a good initial response, with a decrease in his lymph node disease after 3 months. He is continuing with this treatment.
Pathology review. The patient was referred for consultation, and his outside pathology was re-reviewed. Both his original left nephrectomy specimen and retroperitoneal lymph node biopsy specimen were reviewed. The left radical nephrectomy specimen displayed a phenotype that was biphasic, both histologically (Figure 1) and immunochemically (Figure 2), showing a poorly differentiated pT3 medullary-type RCC (Figures 1A and 1B-r) arising next to a pT1 papillary RCC (Figures 1A and 1B-l), which correlated with a collision of two different tumors. The poorly differentiated tumor was consistent with a medullary RCC, and it was this component that showed the aggressive and invasive behavior (Figure 1C), while the papillary component could be interpreted as an incidental, local event. Accompanying immunoperoxidase stains for CK7 and vimentin were positive in both the papillary and poorly differentiated tumors. Staining for carcinoembryonic antigen P (CEA-P) was strongly positive only in the poorly differentiated tumor (Figure 2A). Stains for RCC (Figure 2B), racemase (Figure 2C), and CD10 were strongly positive only in the papillary tumor. Both tumors were negative for high-molecular-weight cytokeratin, cytokeratin 20 (CK20), p53, CD117, desmin, and mucin.
The left periaortic lymph node specimen (Figure 3A) showed a poorly differentiated carcinoma consistent with medullary type RCC. Special immunoperoxidase staining performed in our laboratory showed tumor cells to be positive for vimentin and CEA-P (Figure 3B), and focally positive for CK7. Staining for RCC was negative. Accompanying immunoperoxidase stainings were positive for AE1/AE3, CK7, and vimentin; and negative for CD10, CK20, and thyroid transcription factor 1 (TTF-1). Independent analysis for each tumor marker profile using the ImmunoQuery® database (http://www.immunoquery.com) supported our diagnosis for each tumor. The poorly differentiated component had a high schore (4/5) for a diagnosis of medullary carcinoma and a low score (1/5) for a differential dianosis that included collecting duct and clear-cell–type RCC. However, no ductal morphology was observed in this tumor.
Discussion
Collision tumors occur when two neoplasms (benign and malignant, or both malignant) grow in neighboring anatomic regions and merge. Collision renal tumors are uncommon but have been previously reported and can involve different histologies of kidney carcinoma, such as papillary RCC with oncocytoma,[1] clear-cell RCC and renal pelvis urothelial carcinoma,[2,3] squamous cell carcinoma and osteogenic sarcoma of the kidney,[4] clear-cell RCC with collecting duct carcinoma,[5] and clear-cell RCC with squamous cell carcinoma of the kidney.[6] Renal collision tumors have also been reported in association with other types of cancers, such as breast cancer,[7] prostate cancer,[8] and lymphoma.[9]
Renal medullary carcinoma is a rare, highly aggressive kidney cancer seen most frequently in patients with sickle cell trait or sickle cell hemoglobin SC disease, and is associated with poor response to systemic therapies.[10] In a review of 15 renal medullary carcinoma cases, there appeared to be a male predominance (male-to-female ratio, 2:1), a mean age of 26 years (range, 10–40), and a right kidney predominance (11/15), with positive immunochemistry staining for CEA, CAM5.2, CK7, CK20, AE1/AE3, and vimentin, and absence or heterozygosity of SMARCB1.[11] In our patient’s case, even though he does not have predisposing factors, such as sickle cell trait or sickle cell hemoglobin SC disease, and although he has involvement of the left kidney (instead of the right), the histologic and immunohistochemical evaluations of the poorly differentiated tumor within the collision tumor were consistent with renal medullary carcinoma. Renal medullary carcinomas tend to respond poorly to immunotherapy (α-interferon, interleukin-2) and chemotherapy (cyclophosphamide, doxorubicin, and cisplatin; topotecan and doxorubicin; gemcitabine and cisplatin; single-agent paclitaxel; single-agent vinblastine).[12,13] In single-patient case reports and small retrospective series, potential activity with high-dose-intensity MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) has been reported.[13,14]
The effectiveness of tyrosine kinase inhibitors (TKIs) for non–clear-cell carcinoma, including papillary and medullary RCC, is currently being evaluated. In a series evaluating the molecular characteristics of renal medullary carcinomas, there was strong expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor,[15] suggesting that treatment with VEGF-directed TKIs is feasible. In a phase II trial of sunitinib in 57 patients with advanced non–clear-cell RCC, there were 6 patients with collecting duct or renal medullary histology and 25 patients with papillary histology. While there were no objective responses in either subtype, the stable disease rates were 67% and 48% for the collecting duct/medullary and papillary subtypes, respectively.[16]
Our patient with collision renal papillary and medullary carcinoma has had a good response to TKI therapy with pazopanib thus far, with restaging scans at 3 months demonstrating a decrease in the size of the lymph node disease. He will continue this therapy as long as he continues to respond and continues to tolerate it. Given that his metastatic recurrence is most consistent with the renal medullary carcinoma component of the collision tumor, if he has disease progression in the future, we may consider chemotherapy regimens such as high-dose-intensity MVAC or participation in a clinical trial. Treatment options for renal medullary carcinomas are limited; however, future evaluations of this tumor subtype at the molecular pathology level may inform future therapeutic trials and options.
References:
1. Vasuri F, Fellegara G. Collision renal tumors. Int J Surg Pathol. 2009;17:338-9.
2. Yasada K, Togo Y, Suzuki T, et al. A case of synchronous ipsilateral renal cell carcinoma and renal pelvic urothelial carcinoma (collision tumor): a case report. Hinyokika Kiyo. 2007;53:627-30.
3. Hart AP, Brown R, Lechago J, Truong LD. Collision of transitional cell carcinoma and renal cell carcinoma. An immunohistochemical study and review of the literature. Cancer. 1994;73:154-9.
4. Rothschild J, Bhatt S, Dogra VS. Renal collision tumor in association with xanthogranulomatous pyelonephritis. J Clin Imaging Sci. 2011;1:9.
5. Cho NH, Kim S, Ha MJ, Kim HJ. Simultaneous heterogenotypic renal cell carcinoma: immunohistochemical and karyoptic analysis by comparative genomic hybridization. Urol Int. 2004;72:344-8.
6. Valderrama E, Kalra J, Badlani G, Kahn LB. Simultaneous renal cell carcinoma and squamous cell carcinoma of the kidney. Urology. 1987;29:441-5.
7. Amin M, Radkay L, Pantanowitz L, et al. Tumor-to-tumor metastases (TTM) of breast carcinoma within a solitary renal angiomyolipoma: a case report. Pathol Res Pract. Epub 2013 Jul 3.
8. Vyas M, Menon S, Desai SB. Collision tumor of kidney: a case of renal cell carcinoma with metastases of prostatic adenocarcinoma. Indian J Med Paediatr Oncol. 2013;34:21-3.
9. Wang BY, Strauchen JA, Rabinowitz D, et al. Renal cell carcinoma with intravascular lymphomatosis: a case report of unusual collision tumors with review of the literature. Arch Pathol Lab Med. 2001;125:1239-41.
10. Hakimi AA, Koi PT, Milhoua PM, et al. Renal medullary carcinoma: the Bronx experience. Urology. 2007;70:878-82.
11. Liu QY, Galli S, Srinivasan R, et al. Renal medullary carcinoma: molecular, immunochemistry, and morphologic correlation. Am J Surg Pathol. 2013;37:368-74.
12. Avery RA, Harris JE, Davis CJ, Jr, et al. Renal medullary carcinoma: clinical and therapeutic aspects of a newly described tumor. Cancer. 1996;78:128-32.
13. Rathmell WK, Monk JP. High-dose-intensity MVAC for advanced renal medullary carcinoma: report of three cases and literature review. Urology. 2008;72:659-63.
14. Watanabe IC, Billis A, Guimarães MS, et al. Renal medullary carcinoma: report of seven cases from Brazil. Mod Pathol. 2007;20:914-20.
15. Swartz MA, Karth J, Schneider DT, et al. Renal medullary carcinoma: clinical, pathologic, immunohistochemical, and genetic analysis with pathogenetic implications. Urology. 2002;60:1083-9.
16. Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. 2012;62:1013-9.