Combined-Modality Therapy for Early-Stage Hodgkin Lymphoma: Maintaining High Cure Rates While Minimizing Risks
In Hodgkin lymphoma, as with many other malignancies, a combined-modality approach has proven successful. This tactic capitalizes on the relative advantages of both modalities, yet minimizes risk by avoiding intense exposure to either. This article will summarize the data supporting this approach in early-stage Hodgkin lymphoma.
Multiple randomized studies have demonstrated that chemotherapy, most commonly ABVD (doxorubicin [Adriamycin], bleomycin, vinblastine, dacarbazine), followed by consolidation radiation therapy is the most effective treatment program for early-stage Hodgkin lymphoma. With a combined-modality approach, the great majority of patients are cured of their disease. It is also apparent that both chemotherapy and radiation therapy can increase the risk of complications in the decades following treatment, with second cancers and cardiac disease being the most common. Most studies evaluating such risks primarily include patients treated in decades past with what are now considered outdated approaches, including high-dose, wide-field radiation therapy. The treatment of Hodgkin lymphoma has evolved significantly, particularly in regard to radiation therapy. In combination with chemotherapy, much lower doses and smaller fields are employed, with success equivalent to that achieved using older methods. Many studies have shown a significant decline in both the rates of second cancers and the risk of cardiac disease with low-dose radiation confined to the original extent of disease. In favorable patients, as few as 2 cycles of ABVD have been shown to be effective. The current combined-modality approach seeks to maintain high cure rates but minimize risks by optimizing both chemotherapy and radiation therapy.
Introduction
The great majority of patients with Hodgkin lymphoma are currently cured of their disease using modern treatment regimens. It is extraordinary to recollect that until the mid-20th century this disease was inevitably fatal. In 1950, Vera Peters, MD, from Toronto, published a landmark study demonstrating long-term survival of patients with early-stage disease using radiation therapy alone.[1] After this sentinel observation by Dr. Peters, two concepts emerged from work at Stanford University. The first concept was that a dose-response relationship is seen when radiation is used as the sole modality.[2] The second concept was that the nature of Hodgkin lymphoma is to spread to contiguous lymph nodes.[3] The dose, field size, and treatment techniques were optimized over the ensuing decades using these two concepts. Long-term disease control in the prechemotherapy era was ultimately achieved in 75% to 80% of patients with stage I to II Hodgkin lymphoma using doses of 35 to 44 Gy and large fields (treatment of involved and high-risk adjacent nodal sites). With the development of effective combination chemotherapy in the 1960s, many patients who relapsed after initial radiation therapy were salvaged with chemotherapy; the overall cure rate of early-stage Hodgkin lymphoma with this approach was ~90%.[4]
This was heralded as a notable success, and indeed it was. However, with long follow-up, the risks of this treatment strategy were found to be considerable. In particular, a significant percentage of patients, successfully cured of their Hodgkin lymphoma, subsequently developed a secondary cancer or cardiovascular disease 10 to 25 years after treatment.[5] In the setting of such favorable cure rates, minimizing long-term risks of treatment, both from chemotherapy and radiation therapy, has become a dominant focus in the current design of clinical trials and the management of individual patients.
The goal of any oncologic treatment program is to maximize cure while minimizing risk (eg, acute side effects and late risks). In Hodgkin lymphoma, as with many other malignancies, a combined-modality approach has proven successful. This tactic capitalizes on the relative advantages of both modalities, yet minimizes risk by avoiding intense exposure to either. This article will summarize the data supporting this approach in early-stage Hodgkin lymphoma.
Combined-Modality Therapy: Randomized Trials
TABLE

Randomized Trials Comparing Chemotherapy vs Combined-Modality Therapy
Combination chemotherapy regimens were originally developed primarily for patients with advanced disease. Because of their success in patients with advanced disease, they were later studied for those with localized disease. The initial approach was to investigate the upfront use of chemotherapy and radiation therapy (combined-modality therapy). With high response rates to chemotherapy, however, many queried whether radiation therapy was a necessary component. Several randomized trials were conducted using a variety of chemotherapy regimens and radiation schemes (see Table).[6-14] Despite the heterogeneity, the studies consistently demonstrated a lower risk of relapse with radiation therapy added (Figure 1). Though the individual trials have not shown a consistent effect on survival (and few were powered to detect a survival benefit), a Cochrane systematic review did demonstrate improved overall survival in early-stage disease treated with a combined-modality approach compared with chemotherapy alone.[15]
FIGURE 1
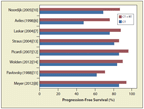
Randomized Trials Comparing Chemotherapy (CT) Plus Radiation Therapy (RT) vs Chemotherapy Alone in Hodgkin Lymphoma
A few of the individual trials are briefly reviewed below to highlight important points.
Picardi et al
This study from University Medical School in Naples, Italy enrolled 160 patients with Hodgkin lymphoma (all with disease ≥ 5 cm).[12] Patients received 6 cycles of VEBEP (vinblastine, etoposide, bleomycin, epirubicin, predisone). Those with a good (≥ 75% reduction in tumor size) but incomplete response by computed tomography (CT) but who were negative by positron emission tomography (PET) were randomized to consolidation radiation therapy (32 Gy) or observation. Eleven patients relapsed in the chemotherapy arm compared with two in the combined modality arm (P = .01). Event-free survival at 5 years was 96% vs 86% (P = .03).
Numerous ongoing studies are evaluating whether PET can distinguish the subgroup of patients who harbor residual disease after chemotherapy and who would therefore gain the most benefit from consolidation radiation therapy. This study is notable because all patients achieved at least a 75% reduction in the areas of bulk disease and a complete response to chemotherapy by PET imaging, but radiation therapy still decreased the risk of relapse. A limitation of this study is the choice of chemotherapy regimen (VEBEP), which may not be optimal based on studies demonstrating that non-doxorubicin (Adriamycin) anthracycline regimens are not as effective as doxorubicin-based regimens.[16]
Patients whose PET and CT scans were both negative (n = 70) in the study by Picardi et al were not randomized and only received chemotherapy. Unfortunately, 10 patients were lost to follow-up. Among the remaining 60, there were still 7 who had relapses. Thus, a negative post-treatment PET scan, even with a negative CT scan, may lack sufficient sensitivity to indicate a true disease-free state. Interim PET, done after 2 to 3 cycles, is also being examined, but interim analyses from a joint study by the European Organisation for Research and Treatment of Cancer (EORTC) and the Groupe d’Etude des Lymphomes de l’Adulte (GELA) study (H10) have not been encouraging.[17]
Wolden et al
This Children’s Cancer Group study (CCG 5942) enrolled 498 children and adolescents who achieved a complete response by CT scan to 6 cycles of chemotherapy (mostly COPP-ABV: cyclophosphamide, vincristine [Oncovin], procarbazine, prednisone, doxorubicin, bleomycin, vinblastine) or the same chemotherapy with radiation therapy (21 Gy).[14] By intent-to-treat analysis, 10-year event-free survival (EFS) was 90% vs 84% (P = .05) in the two groups, respectively. However, 23 patients randomized to radiation therapy did not receive it, and 5 of these patients relapsed. The as-treated EFS values were 91% and 83% for patients randomized to post-chemotherapy radiation vs no further therapy, respectively (P = .004). Among patients relapsing after chemotherapy alone, 85% of relapses were confined to original sites of disease.
The study by Wolden et al highlights several important points. First, quality assurance information is rarely reported in randomized lymphoma trials, making it difficult to ascertain the actual benefit of radiation therapy. Second, congruent with many other studies, patterns of failure confirmed that most recurrences after chemotherapy alone are confined to originally involved areas, supporting the current approach of targeting radiation fields to the original extent of disease. Finally, and perhaps of greatest importance, low doses of radiation therapy are effective after combination chemotherapy, 21 Gy in this study.
Meyer et al
This National Cancer Institute of Canada study (HD6) randomized patients with early-stage, unfavorable Hodgkin lymphoma to 4 to 6 cycles of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or 2 cycles of ABVD plus radiation therapy.[8] The latter consisted of subtotal nodal irradiation to a dose of 35 Gy. Patients with large mediastinal adenopathy, bulky disease (> 10 cm), or intra-abdominal disease were considered high-risk and not eligible for randomization. This study suffers from having two different chemotherapy regimens in the two treatment arms (4 to 6 cycles of ABVD vs 2 cycles), and from what we now know to be a radiation therapy dose and field size with unacceptable risks of complications.
Although freedom from disease progression was improved with combined-modality therapy (94% vs 86%, P = .006), survival was better with ABVD alone. However, imbalances in the number of deaths due to infections (3 vs 0) and miscellaneous causes (5 vs 0), which included Alzheimer’s disease, drowning, suicide, respiratory failure, and unknown cause, are difficult to explain. Further, the number of second cancers outside of a subtotal nodal radiation field (bladder, cervix, prostate, rectal) were higher in the combined-modality arm (n = 6) compared with the chemotherapy alone arm (n = 1).
This study is widely quoted in support of using chemotherapy alone for early-stage Hodgkin lymphoma but is primarily of historical significance, inasmuch as outdated radiation approaches were utilized. In fact, HD6 was closed early after publication of the EORTC H8F trial, which showed that involved-field radiation therapy could replace more extensive radiation fields when chemotherapy was part of the treatment program.[18] Patients in the combined-modality therapy arm were thus exposed to the risks of both chemotherapy and full-dose, wide-field radiation therapy, which may have contributed to the observed late toxicity. It is notable that patients in the combined-modality arm had fewer relapses than patients managed with chemotherapy alone (P = .006).
Combined-Modality Therapy: Optimizing Radiation Therapy
Field size
FIGURE 2
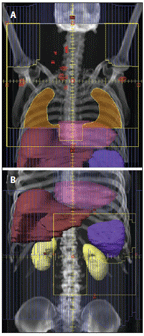
Historical Radiation Fields for Early-Stage, Supradiaphragmatic Hodgkin Lymphoma
Historically, when radiation therapy was utilized as a single modality it was necessary to treat not only known disease but also possible microscopic disease in high-risk adjacent sites. For patients with supradiaphragmatic Hodgkin lymphoma, this most commonly required treatment of all of the lymph node regions above the diaphragm (mantle field) combined with a field encompassing the para-aortic lymph nodes and spleen. This is referred to as subtotal nodal radiation therapy (Figure 2).
In a combined-modality approach, chemotherapy is relied upon to eradicate microscopic disease. Thus, prophylactic coverage of uninvolved sites becomes unnecessary and radiation fields can be significantly reduced. Several randomized trials have confirmed this premise, showing equivalent control rates when involved-field radiation therapy (ie, treatment of the involved lymph node region only) is utilized in lieu of more extensive fields[18-20] (Figure 3A). Involved-field radiation therapy, which has been extensively studied in Hodgkin lymphoma, consists of treatment of an entire region (ie, entire left neck treated even if disease is confined to left supraclavicular fossa).
FIGURE 3
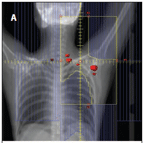
Contemporary Radiation Fields for Early-Stage Hodgkin Lymphoma Used in a Combined-Modality Treatment Program
Even smaller radiation fields are being explored, including involved-site radiation therapy (Figure 3B) and involved-node radiation therapy (Figure 3C). The former consists of treatment of the original extent of disease with a comfortable margin to account for uncertainties in pre- and post-chemotherapy imaging. A retrospective study from British Columbia suggested equivalent outcomes with this approach compared with the standard involved-field method.[21] Involved-node radiation therapy is even more conformal and is confined to the original extent of disease (Figure 3C). International guidelines suggest that radiation treatment planning with PET and CT should be performed both before and after chemotherapy, with careful review of the imaging studies to ascertain original extent of disease and thereby facilitate accurate treatment when using this approach.[22,23]
While such tight fields may decrease the risk of complications, care and judgment are necessary to avoid making radiation fields too small and exposing a patient to an unnecessary risk of relapse. Further, the current use of low doses of radiation therapy (20 Gy) may mean that complications are not significantly improved by modest reductions in field size. It should also be pointed out that randomized trials have not been completed comparing either involved-site or involved-node radiation therapy with involved-field radiation therapy. Currently, either involved-field or involved-site radiation therapy is used in most instances.
Dose
With definitive radiation therapy alone, a dose of 36 to 40 Gy is necessary for clinically evident disease, and a lower dose (26 to 30 Gy) is used for prophylactic treatment areas. After chemotherapy, particularly in the setting of a negative post-treatment PET scan, a much lower dose suffices inasmuch as a dramatically reduced quantity of disease remains. This concept was first proposed by one of us nearly 40 years ago and has subsequently been confirmed by randomized trials.[24]
In a study by the German Hodgkin Study Group (HD1), 20 Gy was compared with 40 Gy in patients with non-bulky presentations after 2 double cycles of chemotherapy (COPP-ABVD).[25] There was no difference in freedom from treatment failure at 4 years (79% vs 80%). A randomized study by the GOELAMS (Groupe Ouest Est d’tude des Leucnies et Autres Maladies du Sang) Hodgkin Lymphoma Network in France (H97-E) also confirmed that lower doses are sufficient with a combined-modality approach.[26] Final results of the EORTC-GELA H9 trial, which compared no RT (closed early due to an unacceptable failure rate), 20 Gy, and 36 Gy are awaited.[10]
FIGURE 4

Freedom From Treatment Failure in the German Hodgkin Study Group HD10 Trial
An important recent study from the German Hodgkin Study Group (HD10) randomized 1370 patients with early-stage, favorable Hodgkin lymphoma to 20 Gy vs 30 Gy of radiotherapy after 2 to 4 cycles of ABVD.[27] Favorable disease was defined as two or fewer sites of involvement, nonbulky tumors, no extranodal disease, and a favorable B-symptoms/erythrocyte sedimentation rate (ESR) ratio. There was no difference in the primary endpoint, freedom from treatment failure (92.9% vs 93.4%, P = 1), or in any secondary endpoints, including overall survival, between the two arms (Figure 4). Thus, for patients with early-stage, favorable Hodgkin lymphoma, 20 Gy of radiation therapy after 2 cycles of ABVD is sufficient.
For patients with early-stage, unfavorable Hodgkin lymphoma there remains some uncertainty and practice variability. The German Hodgkin Study Group HD11 study randomized 1395 patients to 4 cycles of either ABVD or non-escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone).[28] A secondary randomization was to 20 Gy or 30 Gy of consolidation radiation therapy. While 20 Gy appeared as effective as 30 Gy with BEACOPP, a clinically relevant inferiority of 20 Gy could not be excluded with ABVD. The authors concluded that 4 cycles of ABVD should be followed by 30 Gy of radiation therapy.
Data from our institution suggest that 20 Gy after 6 cycles of chemotherapy for unfavorable Hodgkin lymphoma is effective and sufficient in the presence of negative post-chemotherapy functional imaging (gallium scans, in this study).[29] These data were obtained principally from patients with large mediastinal adenopathy. The assumption that 6 cycles of ABVD + 20 Gy of radiation therapy is equally effective and less toxic than 4 cycles of ABVD + 30 Gy needs to be further studied.
Smaller fields and lower doses: implications for risk
It is intuitive that treating fewer areas with lower doses of radiation therapy will be associated with fewer long-term risks. The more critical question is the magnitude of the risk using modern radiation techniques. This may take many more years to fully recognize, since some effects are not manifest for many decades after treatment. However, several studies have suggested that risks are significantly less with current treatment approaches.[4,20,30-38]
Secondary Malignancies
It has been conclusively demonstrated that patients treated for Hodgkin lymphoma are at higher risk for the development of second cancers compared with an age-matched healthy population. The most common second malignancies include female breast cancer and lung cancer.[39] The risk of breast cancer is elevated many years before routine screening is recommended in the general population, which has implications for post-treatment surveillance. Although factors such as genetic predisposition and immunodeficiency may be contributing, the majority of excess second cancers are thought to be treatment-related.
Most studies have shown lower rates of second cancers with combined-modality therapy compared with definitive radiation therapy. Koontz et al evaluated 111 patients from Duke Cancer Institute treated with either definitive radiation therapy (subtotal nodal fields; median dose, 38 Gy) or combined modality therapy (involved fields; median dose, 26 Gy).[4] The 20-year actuarial risk of developing a second cancer was 16% with definitive radiation therapy alone and 0% with combined-modality therapy. A similar decline in the incidence of secondary malignancies with low-dose, involved-field radiation therapy was observed in a study by investigators from Yale University School of Medicine.[36]
Specifically evaluating radiation field size, the German Hodgkin Study Group trial HD8 randomized early-stage favorable patients following chemotherapy to consolidation radiation therapy using either an extended-field or an involved-field approach. As referenced previously, there was no difference in outcomes with smaller radiation fields.[18] Long-term follow-up did demonstrate a decline in all intercurrent illness mortality (12.2% vs 15%). Further, there was a reduction in the number of deaths due to secondary malignancies (45 vs 58), particularly acute myeloid leukemia and myelodysplasia (4 vs 11).[37] Long-term results of a randomized study from Milan comparing subtotal nodal vs involved-field radiation therapy after ABVD also showed fewer secondary malignancies with smaller radiation fields (0 vs 3).[20] Finally, De Bruin et al from the Netherlands observed a significantly lower risk of breast cancer in female patients treated with radiation to the mediastinum alone as opposed to a full mantle field (which includes the axillary regions and necessarily more breast tissue).[31]
In the pediatric literature, lower doses have also been associated with a lower risk of second cancers in some,[30,34] but not all,[40] studies. In the study from Stanford,[40] the 20-year actuarial risk of developing a secondary cancer was 17%, similar to historical values, with breast cancer and thyroid cancers being most common. Thyroid cancers are known to be associated with low radiation doses in the pediatric population. Longer follow-up is necessary, both in the adult and pediatric populations, to ascertain risks associated with smaller radiation fields and lower doses.
Cardiac Complications
Cardiac disease is the second most prevalent late effect, behind secondary malignancies, in Hodgkin survivors. Since radiation therapy can affect all of the structures of the heart, the complications that have been observed include coronary artery disease leading to myocardial infarction, valvular disease, pericardial disease, conduction abnormalities, and cardiomyopathies. Pericarditis was a common complication in the early days of radiation therapy when the entire heart was treated with relatively high doses, but it is rarely observed today.
While mediastinal radiation therapy clearly increases the risk of developing heart disease, anthracyclines appear to compound this risk, particularly for congestive heart failure and valvular disorders.[41,42] Thus, minimizing both radiation and chemotherapy exposure is important. Further, patients with known cardiac risk factors (eg, diabetes, hypertension, hyperlipidemia, smoking) appear to be at much higher risk for the development of cardiac complications compared with patients with no cardiac risk factors.[33,43] Thus, mediastinal radiation therapy can be considered a cardiac risk factor, which when compounded by diabetes or other cardiac comorbidities, begins to increase the risk of an adverse event. Follow-up appointments should emphasize regular exercise, a balanced diet, maintaining a healthy weight, tobacco avoidance, and other lifestyle measures that will decrease the risk of cardiac disease in the ensuing decades.
Cardiac complications are clearly related to the dose and field of radiation. Many studies have shown that lower radiation doses are associated with a lower risk of cardiac disease.[32,33,35] In a study of 1132 Hodgkin survivors treated before they were 18 years of age, the 25-year actuarial risk of cardiac disease was 21% after they had received a mediastinal dose of 36 Gy compared with 3% when 20 Gy was utilized (P < .001).[38] Radiation fields that are more conformal to the disease in the mediastinum are able to avoid the valves and coronary arteries in many patients with Hodgkin lymphoma. Geographic avoidance is the best way to decrease the risk of complications.
Combined-Modality Therapy: Optimizing Chemotherapy
Acute and long-term toxicity of chemotherapy is less commonly discussed in the context of optimizing treatment for Hodgkin lymphoma. Just as radiation therapy carries inherent risks, cytotoxic drugs do as well. Intensive multi-agent chemotherapy regimens increase the risk of cardiac dysfunction, pulmonary complications, neuropathy, infertility, and secondary cancers. Arguably, late effects of radiation therapy have been more comprehensively studied than late effects from chemotherapy. The optimal number of cycles and the best chemotherapeutic regimen are areas of active investigation, with the goal of maximizing the chance of cure while minimizing side effects.
The German Hodgkin Lymphoma Group HD10 study clearly shows that, when followed by radiation therapy, 2 cycles of ABVD are as effective as 4 cycles in patients with early-stage, favorable Hodgkin lymphoma.[27] Two cycles of ABVD were less toxic than 4 cycles, with grade 3/4 adverse events occurring in 33% vs 52% of patients, respectively (P < .001). Fewer infections (10 vs 30), respiratory tract disorders (2 vs 12), deaths due to ABVD toxicity (0 vs 6), and deaths due to cardiovascular events (3 vs 6) were observed. No difference in development of secondary cancers has been reported (4.5% vs 4.7%), but follow-up is short.
More intense combined modality therapy may be required to effectively treat patients with early-stage, unfavorable Hodgkin lymphoma. Whether optimal chemotherapy in this high-risk group should be ABVD or BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone), is under debate.
As previously mentioned, the HD11 trial had a 2 × 2 factorial design that compared 20 Gy vs 30 Gy of radiation therapy and 4 cycles of ABVD vs 4 cycles of standard BEACOPP.[28] BEACOPP did not improve clinical outcomes but was associated with more toxicity. Grade 3/4 toxicity occurred in 74% vs 52% (P < .001) of patients treated with BEACOPP vs ABVD, respectively, leading to a higher rate of hospitalization (59% vs 43%, respectively). Therefore, the conclusion of this study was that 4 cycles of ABVD plus 30 Gy of radiation therapy should be the standard of care for early-stage, unfavorable Hodgkin lymphoma.
In an attempt to further improve long-term control of Hodgkin lymphoma in patients with early-stage, unfavorable disease, the German Hodgkin Study Group HD14 trial was developed. This was a randomized study of 4 cycles of ABVD vs 2 cycles of escalated BEACOPP + 2 cycles of ABVD with radiation therapy (at 30 Gy) administered in both arms.[44] The 5-year progression-free survival was statistically significantly higher in the BEACOPP arm (at 95.4% vs 89.1% with ABVD plus radiotherapy, (P < .001) but the 5-year overall survival was 97% in both arms. Grade 3/4 hematologic adverse events were more frequent with BEACOPP (87% vs 51%, respectively). No increased incidence of secondary malignancy or long-term toxicity has been demonstrated thus far, but follow-up is short.
The longer progression-free survival seen with the addition of escalated BEACOPP to ABVD has led the German Hodgkin Study Group to recommend this as standard therapy for early-stage, unfavorable Hodgkin lymphoma. However, the overall survival is identical with ABVD and BEACOPP chemotherapy regimens, likely due to the efficacy of salvage regimens for relapsed disease. For now, ABVD continues to be the standard of care in the United States.
Future areas of investigation involve the incorporation of new drugs into the standard chemotherapy regimens, such as the use of the anti-CD30 monoclonal antibody brentuximab vedotin (Adcetris) as a substitute for bleomycin in an ABVD-like regimen; the study of 6 cycles of ABVD in combination with 20 Gy of radiation therapy for early-stage unfavorable disease; and investigation of whether mid-treatment PET scans can risk-stratify patients and determine which patients, if any, can stop treatment after fewer cycles of chemotherapy.
Combined-Modality Therapy: An Optimal Balance
A combination of chemotherapy and radiation therapy provides optimal disease control in early-stage Hodgkin lymphoma. The risks of treatment will continue to decline as both modalities are refined. While results with chemotherapy alone are good, they remain inferior to a combined modality approach. Administering more-intense chemotherapy in an attempt to avoid radiation therapy may prove counterproductive from the perspectives of both disease control and late toxicity. High-dose chemotherapy and stem cell transplant can salvage some patients who relapse after receiving chemotherapy alone. However, this is successful in only a fraction of patients, and long-term morbidity from high-dose chemotherapy must also be considered. As the disciplines of both radiation and medical oncology work together to optimize their individual contributions to the management of early-stage Hodgkin lymphoma, high cure rates will be maintained and long-term morbidity will be minimized.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Peters MV. A study of survivals in Hodgkin’s disease treated radiologically. Am J Roentgenol Radium Ther. 1950;63:299-311.
2. Kaplan HS. Evidence for a tumoricidal dose level in the radiotherapy of Hodgkin’s disease. Cancer Res. 1966;26:1221-4.
3. Rosenberg SA, Kaplan HS. Evidence for an orderly progression in the spread of Hodgkin's disease. Cancer Res. 1966;26:1225-31.
4. Koontz BF, Kirkpatrick JP, Clough RW, et al. Combined-modality therapy versus radiotherapy alone for treatment of early-stage Hodgkin's disease: cure balanced against complications. J Clin Oncol. 2006;24:605-11.
5. Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol. 2002;20:2101-8.
6. Aviles A, Delgado S. A prospective clinical trial comparing chemotherapy, radiotherapy and combined therapy in the treatment of early stage Hodgkin’s disease with bulky disease. Clin Lab Haematol. 1998;20:95-9.
7. Laskar S, Gupta T, Vimal S, et al. Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: is there a need? J Clin Oncol. 2004;22:62-8.
8. Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;
366:399-408.
9. Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20:3765-71.
10. Noordijk EM, Thomas J, Ferme C, et al. First results of the EORTC-GELA H9 randomized trials: the H9-F trial (comparing 3 radiation dose levels) and H9-U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin’s lymphoma (HL). J Clin Oncol. 2005;23(16S): abstr 6505.
11. Pavlovsky S, Maschio M, Santarelli MT, et al. Randomized trial of chemotherapy versus chemotherapy plus radiotherapy for stage I-II Hodgkin’s disease. J Natl Cancer Inst. 1988;80:1466-73.
12. Picardi M, De Renzo A, Pane F, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin’s lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk Lymphoma. 2007;48:1721-7.
13. Straus DJ, Portlock CS, Qin J, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood. 2004;104:3483-9.
14. Wolden SL, Chen L, Kelly KM, et al. Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with hodgkin’s lymphoma--a report from the Children’s Oncology Group. J Clin Oncol. 2012;
30:3174-80.
15. Franklin JG, Paus MD, Pluetschow A, et al. Chemotherapy, radiotherapy and combined modality for Hodgkin’s disease, with emphasis on second cancer risk. Cochrane Database Syst Rev. 2005(4):
CD003187.
16. Pavone V, Ricardi U, Luminari S, et al. ABVD plus radiotherapy versus EVE plus radiotherapy in unfavorable stage IA and IIA Hodgkin’s lymphoma: results from an Intergruppo Italiano Linfomi randomized study. Ann Oncol. 2008;19:763-8.
17. Engert A. Radiotherapy in early-stage Hodgkin lymphoma. The ASCO Post. June 15, 2012.
18. Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601-8.
19. Zittoun R, Audebert A, Hoerni B, et al. Extended versus involved fields irradiation combined with MOPP chemotherapy in early clinical stages of Hodgkin’s disease. J Clin Oncol. 1985;3:207-14.
20. Bonadonna G, Bonfante V, Viviani S, et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin’s disease: long-term results. J Clin Oncol. 2004;22:2835-41.
21. Campbell BA, Voss N, Pickles T, et al. Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin’s lymphoma: a question of field size. J Clin Oncol. 2008;26:5170-4.
22. Eich HT, Muller RP, Engenhart-Cabillic R, et al. Involved-node radiotherapy in early-stage Hodgkin’s lymphoma. Definition and guidelines of the German Hodgkin Study Group (GHSG). Strahlenther Onkol. 2008;184:406-10.
23. Girinsky T, Specht L, Ghalibafian M, et al. The conundrum of Hodgkin lymphoma nodes: to be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol. 2008;88:202-10.
24. Prosnitz LR, Farber LR, Fischer JJ, et al. Low dose radiation therapy and combination chemotherapy in the treatment of advanced Hodgkin’s disease. Radiology. 1973;107:187-93.
25. Loeffler M, Diehl V, Pfreundschuh M, et al. Dose-response relationship of complementary radiotherapy following four cycles of combination chemotherapy in intermediate-stage Hodgkin’s disease. J Clin Oncol. 1997;15:2275-87.
26. Arakelyan N, Jais JP, Delwail V, et al. Reduced versus full doses of irradiation after 3 cycles of combined doxorubicin, bleomycin, vinblastine, and dacarbazine in early stage Hodgkin lymphomas: results of a randomized trial. Cancer. 2010;116:4054-62.
27. Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640-52.
28. Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199-206.
29. Elconin JH, Roberts KB, Rizzieri DA, et al. Radiation dose selection in Hodgkin's disease patients with large mediastinal adenopathy treated with combined modality therapy. Int J Radiat Oncol Biol Phys. 2000;48:1097-105.
30. Constine LS, Tarbell N, Hudson MM, et al. Subsequent malignancies in children treated for Hodgkin’s disease: associations with gender and radiation dose. Int J Radiat Oncol Biol Phys. 2008;72:24-33.
31. De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239-46.
32. Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270:1949-55.
33. Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831-7.
34. Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27:3901-7.
35. Kupeli S, Hazirolan T, Varan A, et al. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin’s lymphoma. J Clin Oncol. 2010;28:1025-30.
36. Salloum E, Doria R, Schubert W, et al. Second solid tumors in patients with Hodgkin’s disease cured after radiation or chemotherapy plus adjuvant low-dose radiation. J Clin Oncol. 1996;14:2435-43.
37. Sasse S, Klimm B, Görgen H, et al. Comparing long-term toxicity and efficacy of combined modality treatment including extended- or involved-field radiotherapy in early-stage Hodgkin’s lymphoma. Ann Oncol. 2012;23:2953-9.
38. Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55:1145-52.
39. Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol. 2007;25:1489-97.
40. O’Brien MM, Donaldson SS, Balise RR, et al. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol. 2010;28:1232-9.
41. Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878-86.
42. Myrehaug S, Pintilie M, Tsang R, et al. Cardiac morbidity following modern treatment for Hodgkin lymphoma: supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma. 2008;49:
1486-93.
43. Glanzmann C, Kaufmann P, Jenni R, et al. Cardiac risk after mediastinal irradiation for Hodgkin’s disease. Radiother Oncol. 1998;46:51-62.
44. von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin study group HD14 trial. J Clin Oncol. 2012;30:907-13.