Prostate Cancer: What Did We Learn From the 2012 Annual Scientific Meeting of ASCO?
This review summarizes recent findings in clinical prostate cancer research reported at the 2012 Annual Scientific Meeting of the American Society of Clinical Oncology (ASCO) and addresses their relevance to clinical practice.
In recent years, management of prostate cancer has benefited from an expanding array of new pharmacological options that are the result, in part, of an increased understanding of, and ability to target, androgen signaling. Further characterization of additional molecular pathways relevant to prostate cancer tumorigenesis has identified new molecular targets in prostate cancer that may be amenable to therapeutic intervention with novel agents. In addition, novel radioisotope therapies and cytotoxic agents have shown significant anticancer efficacy with manageable patterns of toxicity, and will eventually have to be integrated into our algorithms of therapy. This review summarizes recent findings in clinical prostate cancer research reported at the 2012 Annual Scientific Meeting of the American Society of Clinical Oncology (ASCO) and addresses their relevance to clinical practice.
Initial Hormonal Therapy in Metastatic Disease
Inhibition of androgen signaling remains a core treatment strategy in patients with prostate cancer. Although first-line androgen-deprivation therapy (ADT) with luteinizing hormone-releasing hormone analogs (LHRHa) remains standard in patients with metastatic castrate-sensitive disease, the optimal schedule of ADT has been debated. Continuous androgen deprivation (CAD) leads to skeletal and cardiovascular complications as well as to decrements in quality of life due to impaired sexual function. Intermittent androgen deprivation (IAD) in castrate-sensitive patients has been pursued in an attempt to lessen the adverse effects of ADT, although the impact on disease and overall survival (OS) with this approach has not been clearly defined.[1] Randomized trials have suggested IAD has no detrimental impact on OS in this patient population, although these trials have been insufficiently powered to draw firm conclusions.[2-4]
In the early 1990s, the Southwest Oncology Group (SWOG) trial SWOG 9346 (INT-0162) was designed as a randomized phase III study to assess the impact on overall survival of IAD vs CAD in patients with androgen-dependent, metastatic disease. The final results of this trial were reported in a plenary session at the 2012 annual scientific meeting of the American Society of Clinical Oncology (ASCO).[5] Patients with measurable disease and a prostate-specific antigen (PSA) level ≥ 5 ng/mL initially received 7 months of continuous, combined androgen blockade with a LHRH-agonist and an antiandrogen. A total of 3040 patients were accrued to this trial, and 1535 patients achieved a PSA level ≤ 4 ng/mL after the induction phase of CAD. Of this latter group of responding patients, 759 were randomized to remain on continuous androgen deprivation while 770 patients transitioned to IAD. After a median follow-up of 9.2 years, median OS trended towards benefit in the CAD arm following randomization (5.8 vs 5.1 years), but failed to reach statistical significance for the entire study population (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.95 to 1.24). Subset analysis revealed that in patients with extensive disease (defined as involvement of ribs, long bones, and/or visceral organs), IAD was not inferior; however, in patients with minimal disease (defined as involvement of spine, pelvis, and/or lymph nodes), CAD was statistically superior to IAD. In this subset, the median OS times for patients on the CAD and IAD arms were 7.1 and 5.2 years, respectively (HR, 1.23; 95% CI, 1.02 to 1.49, P = .034). The authors concluded that CAD remains preferable to IAD, particularly in patients with minimal disease. Of note, detailed discussion of the patterns of toxicity will form the basis of a future publication from these investigators. The view of the authors regarding superiority of CAD seems to be a very reasonable position, although it is important to note that there was considerable controversy and discussion in a planned session at the 2012 ASCO Annual Meeting. In this session, it was noted that the difference in outcome overall was not very large, and it was suggested that toxicity is significantly less among patients on IAD. Thus an alternative view, which we favor, is that it may be preferable to share the information with patients, particularly those with extensive disease, explaining the amplitude of the difference in survival, accompanied by a discussion of potential toxic effects of CAD and IAD. This would allow each patient to make an informed decision predicated on the balance between efficacy and survival vs the toxicity of treatment, allowing patients to choose a treatment that best reflects their own imperatives and concerns.
Salvage Hormonal Therapy
In the castrate-resistant setting, recent clinical progress has been achieved by therapeutic targeting of extragonadal androgen synthesis and more potent inhibition of androgen-receptor signaling. The enzyme CYP17 is responsible for androgen production in the adrenal glands. Weak inhibition of this enzyme by ketoconazole demonstrates modest activity in patients with castrate-resistant disease,[6] which sometimes may be sustained for many months.
TABLE
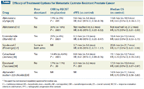
Efficacy of Treatment Options for Metastatic Castrate-Resistant Prostate Cancer
Abiraterone acetate (Zytiga), developed as a more potent inhibitor of CYP17, reduces systemic androgens to undetectable levels when used in combination with an LHRHa.[7] Complete ablation of systemic androgens in the castrate setting has proven beneficial, as shown by a 3.9-month OS benefit for treatment with abiraterone and prednisone compared with prednisone alone in patients previously treated with docetaxel (Taxotere) (Table).[8] Currently, docetaxel is the only agent approved for initial use in patients with mCRPC; however, use of docetaxel in this setting is often limited by declining patient fitness or preference, as well as by occasional patterns of excessive toxicity vs clinical benefit, thus there is much interest in developing more effective second-line hormonal therapies in the chemonaive setting.
An early-phase trial of abiraterone in the chemonaive setting demonstrated antitumor activity,[9] prompting design of the COU-AA-302 trial, which is a multinational randomized phase III study evaluating the efficacy of abiraterone in mCRPC patients prior to their receiving docetaxel-based therapy. A total of 1088 patients with minimal or no symptoms were randomized to receive abiraterone with prednisone vs placebo with prednisone. Following a preplanned interim analysis of results after a median follow-up of 22.2 months, the study was unblinded as recommended by the data monitoring committee after abiraterone demonstrated benefit in all endpoints.[10] OS favored abiraterone over placebo (not reached [NR] vs 27.2 months, respectively: HR, 0.75; 95% CI, 0.61 to 0.93; P = .0097), and the co-primary endpoint of radiographic progression-free survival (rPFS) also showed significant benefit of abiraterone over placebo (NR vs 8.3 months, respectively: HR, 0.43; 95% CI, 0.35 to 0.52; P < .0001). The overall response rate (ORR) of the abiraterone cohort was 36% vs 16% in the placebo arm (P < .0001), a particularly important point that illustrates the innate heterogeneity of clinical progression of this disease. Furthermore, time to initiation of chemotherapy was delayed by 8.4 months in the group receiving abiraterone. These results potentially support a role for abiraterone in the chemonaive setting, although regulatory approval for this indication has not yet been granted.
Additional targeting of the androgen axis has resulted from development of more potent androgen receptor (AR) antagonists. Enzalutamide (Xtandi) binds ARs with higher affinity than bicalutamide (Casodex), leading to greater inhibition of AR signaling.[11] Based on promising early-phase studies,[12] the AFFIRM trial randomized 1199 men with mCRPC previously treated with docetaxel 2:1 to receive enzalutamide vs placebo. Following preplanned interim analysis, the study was unblinded early due to a marked benefit in the enzalutamide arm.[13] On the basis of superior median OS favoring enzalutamide (18.4 months vs 13.6 months; HR, 0.631; 95% CI, 0.529 to 0.752; P < .001), the United States Food and Drug Administration (FDA) approved enzalutamide for use in docetaxel-treated men with mCRPC on August 31, 2012. The rPFS was significantly prolonged in the enzalutamide arm (8.3 vs 2.9 months; HR, 0.404; 95% CI, .350 to .466; P < .0001), and the ORR was 28.9% vs 3.9% in the placebo arm. Like abiraterone, enzalutamide is also anticipated to have activity in the pre-docetaxel setting, although this question is the subject of the ongoing PREVAIL trial (National Cancer Institute [NCI] Clinical Trials.gov identifier: NCT01212991).
The success of abiraterone and enzalutamide have renewed our interest in targeting androgen signaling in mCRPC. With these new agents, clinicians are challenged with how best to use them in practice to optimize patient outcomes. Little is known about the efficacy of these agents when used in sequence, and whether synergism may exist when they are used in combination or with traditional cytotoxic agents. Because abiraterone and enzalutamide disrupt androgen signaling through different mechanisms, cross-resistance may not be universal. This is suggested by the findings from a small retrospective series of 24 patients with mCRPC treated with abiraterone who had progressed following treatment with docetaxel and enzalutamide.[14] In this series, 13% of patients experienced a PSA decline of > 50%, and 29% experienced a reduction in pain and decreased analgesic use, suggesting a modest benefit to sequential use of these new agents. We believe that a more promising approach will be the use of these agents in combination, which is the focus of an ongoing phase II study (NCT01650194).
The landscape of treatment options for advanced prostate cancer is evolving quickly following recent approval of multiple new agents. Defining how best to use these agents is the subject of many ongoing clinical trials and will likely be an area of active clinical research for many years. Furthermore, development of additional compounds targeting androgen signaling continues, including AR antagonist ARN-509, which has demonstrated encouraging activity in a phase I trial[15] and is currently being evaluated in the phase II setting (NCT01171898). Orteronel (TAK-700) is a novel CYP17 inhibitor, similar to abiraterone, that has also demonstrated encouraging early-phase activity[16] and is also being evaluated in the phase III setting (NCT01193257, NCT01193244).
Hormonal Therapy in Nonmetastatic Disease
Docetaxel improves survival in patients with mCRPC, although it is unknown whether adjuvant chemotherapy is beneficial to high-risk patients following prostatectomy. The TAX-3501 trial, which randomized high-risk patients to immediate or delayed docetaxel following prostatectomy, closed prematurely due to poor accrual.[17] This reflects waning enthusiasm from patients and physicians alike for the use of traditional cytotoxic agents in the perioperative setting given the increasing availability of more effective options for hormonal therapy, and also the demonstration of unexpected toxicity from adjuvant mitoxantrone in the SWOG 9921 trial.[18]
In the neoadjuvant setting, ADT has not shown conclusive benefit to date, which may be due in part to persistent intraprostastic androgens,[19] or perhaps is a function of relatively short durations of induction therapy or heterogeneity of receptor expression in many cases.[20] Since the combination of abiraterone and LHRHa can achieve undetectable systemic androgen levels,[7] the impact on intraprostatic androgen levels is also being investigated.[21,22] Taplin et al reported preliminary findings of a phase II neoadjuvant study assessing the use of preoperative abiraterone and LHRHa.[22] All 58 patients received preoperative LHRHa therapy for 24 weeks and concurrent abiraterone during the second 12 weeks prior to radical prostatectomy; 30 of the 58 patients also received concurrent abiraterone during the entire 24-week preoperative period. All patients underwent re-biopsy after 12 weeks for measurement of the impact on intraprostatic androgens, which has not yet been reported. In the initial report, 1 patient (4%) in the LHRHa-only arm achieved a PSA nadir ≤ 0.2 ng/dL compared with 26 patients (90%) in the group receiving both LHRHa and abiraterone after the first 12 weeks of neoadjuvant hormonal therapy. Interestingly, this difference was overcome during the final 12 weeks of preoperative therapy following the addition of abiraterone to the cohort that received only LHRHa therapy initially. The proportion of patients achieving a PSA nadir ≤ 0.2 ng/dL was similar in both groups after 24 weeks total (85% vs 86%). Despite the PSA response, the duration of maximal androgen blockade may be more important to clinical outcome, as the group receiving abiraterone for the entire 24-week preoperative period attained a 34% total or near complete response (CR) rate compared with 15% in the group who received abiraterone only for the final 12-week period (P = .0894).
Preliminary results were also reported by Efstathiou et al for a similar study of neoadjuvant abiraterone and LHRHa.[21] In this randomized phase II trial, patients with high-risk local prostate cancer were randomized 2:1 to receive neoadjuvant abiraterone and LHRHa vs LHRHa alone for 12 weeks before prostatectomy. Primary endpoints include pathologic down-staging and safety. Interim analysis of 37 patients demonstrated that the group receiving both agents had a greater likelihood of undetectable preoperative PSA (68% vs 0%, P = .0001), a surprising result in our experience and the reports of others. Near complete cytoreduction, defined as < 6 mm of scattered cells, also favored patients receiving both agents (24% vs 8%), and pT2N0 pathologic staging occurred in more patients treated with both agents (60% vs 33%, P = .17). A similar phase II neoadjuvant trial is planned with enzalutamide (NCT01547299). These findings suggest neoadjuvant abiraterone may enhance the tumoricidal effect of LHRHa therapy, although whether this will translate into meaningful clinical benefit remains to be seen and will likely require longer durations of perioperative hormonal therapy.
At present, these approaches must be viewed as investigational until there is Level 1 evidence to show that neoadjuvant hormonal therapies, either with conventional approaches or incorporating some of the novel agents and regimens reported at ASCO, improve OS. At the Levine Cancer Institute, our standard off-protocol is to stage patients with high-risk disease carefully, and to use regimens supported by Level 1 evidence for patients with newly diagnosed prostate cancer, focusing either on combined modality hormones and radiation or on radical prostatectomy with adjuvant hormonal therapy for patients with local spread.
New Treatments for Bone Metastases
Development of symptomatic bone metastases commonly affects patients with mCRPC. Zoledronic acid (Zometa) and denosumab (Xgeva) reduce disease-related skeletal morbidity and are widely utilized in patients with bone metastases.[23] Beta-particle–emitting radiopharmaceuticals including samarium-153 and strontium-89 can provide analgesia for painful bone metastases, although the use of these agents is often limited by myelosuppression.[24,25] Alpha-emitting radium-223 (Ra-223, Alpharadin) is a novel bone-seeking radiopharmaceutical that may cause less myelotoxicity than beta-emitters due to the shorter distance travelled by alpha-particles. In a randomized phase II study comparing Ra-223 vs placebo in patients with mCRPC and bone pain requiring external beam radiotherapy, Ra-223 was associated with a nonsignificant trend towards delay in time to a skeletal-related event (SRE) and improvement in OS, with no increased hematologic toxicity relative to placebo.[26] Based on these findings, the multinational phase III ALSYMPCA trial compared Ra-223 to placebo in patients with mCRPC and ≥ 2 bone metastases, who had previously received, declined, or were unfit to be treated with docetaxel.[27] Patients were randomized 2:1 to receive Ra-223 or placebo every 4 weeks for 6 cycles. Based on an updated interim analysis of 921 patients in the trial, Ra-223 prolonged median OS relative to placebo (14.9 vs 11.3 months; HR, 0.695; 95% CI, 0.581 to 0.832; P = .00007). Ra-223 also delayed the time to first SRE (15.6 vs 9.8 months; HR, 0.658; 95% CI, 0.522 to 0.830; P = .00037). Consistent with early-phase studies, myelosuppression was only slightly more prevalent in patients who received Ra-223. Grade 3/4 neutropenia was observed in 2.2% vs 0.7% of patients receiving placebo. Grade 3/4 thrombocytopenia occurred in 6.3% of the Ra-223 group vs 2% of placebo patients. Based on these results, Ra-223 has been submitted for regulatory review and seems likely to be another pharmacologic option with a proven survival benefit available to mCRPC patients, including heavily pretreated patients, in the near future. Subject to regulatory approval, we anticipate adding this option to our armamentarium, but we will carry out future studies to determine where it should fit in the therapeutic sequence. Given the purported low-level myelotoxicity, we anticipate that it will replace samarium and strontium in routine use, although Level 1 evidence will also be required to support any change in treatment standard.
Novel Agents
Another avenue of progress has been the identification of other molecular pathways in prostate tumorigenesis that have led to the discovery of novel therapeutic targets. MET is a receptor tyrosine kinase associated with oncogenic signaling that is expressed in prostate cancer and bone metastasis and is negatively regulated by androgen signaling.[28,29] Inhibition of MET activity has proven effective in murine xenograft prostate cancer models.[30] Cabozantinib (XL-184) is a novel multi–tyrosine-kinase inhibitor with strong activity against MET and vascular endothelial growth factor (VEGF receptor) 2.[31] A randomized phase II study of cabozantinib in patients with mCRPC initially reported at ASCO last year was prematurely unblinded based on significant clinical activity.[32] A total of 86% of patients with lesions evaluable on bone scan demonstrated complete or partial resolution of skeletal lesions. In a study of an expanded nonrandomized cohort of 93 patients previously treated with docetaxel, reported this year,[33] 60% of patients demonstrated a partial response on bone scan, and 46% of patients had reduced narcotic requirements following treatment with cabozantinib. In 59 patients with ≥ 5 circulating tumor cells (CTCs) prior to therapy, 39% converted to < 5 CTCs after at least 12 weeks of therapy, which may suggest a potential survival benefit in this subset.[34] The most common severe adverse events were fatigue and nausea, occurring in 19% and 10% of patients in this cohort, respectively. On the basis of these encouraging findings, phase III trials of cabozantinib in patients with mCRPC are in progress (NCT01605227, NCT01522443).
Placing Data Into Context
The management of advanced prostate cancer has evolved considerably since the development of combined androgenic blockade and the introduction of cytotoxics into routine management. Treatment options associated with prolongation of survival now include new cytotoxic agents (cabazitaxel), immunotherapy (sipuleucel-T [Provenge]), and more effective hormonal blocking agents (abiraterone, enzalutamide), with the prospect of additional compounds available in the near future.
The expanding array of available agents to treat advanced prostate cancer poses a challenge to practitioners regarding how best to sequence available treatment options. Currently, for patients with symptomatic, castrate-resistant disease, we suggest treatment with docetaxel, which remains the standard first-line option. In patients who clinically improve following docetaxel, treatments maximizing additional endocrine therapy with abiraterone or enzalutamide are sensible next options at progression. Alternately, fit patients, especially those less than 65 years of age, who remain symptomatic and who fail to benefit meaningfully from docetaxel, may be considered for cabazitaxel prior to further endocrine therapy. For symptomatic, less fit, or elderly patients who have progressed following docetaxel, abiraterone or enzalutamide should be the next-utilized agents.
The efficacy and tolerability of newer hormonal therapies provide potential new options to clinicians managing advanced disease. Although the use of abiraterone prior to docetaxel for CRPC is not currently supported by regulatory approval, the results of COU-AA-302 appear to show the benefit of more active hormonal therapies in the chemonaive setting, without a major treatment-related decrement in quality of life. Given the favorable toxicity profile of abiraterone relative to available cytotoxic agents, we anticipate that the primary role of abiraterone and potentially enzalutamide will likely shift to the chemonaive setting, especially in older less fit patients, pending future regulatory approval and the results of ongoing trials.
Findings reported at the 2012 ASCO Annual Meeting reflect the pace of clinical research in prostate cancer and continue to support the need for referral of patients into well-structured clinical trials, rather than recycling patients through a series of moderately ineffective, established treatments.
One of the issues that will bear further consideration is the migration of stage and outcome assessment that has occurred over the past decades,[35] and we must be careful not to overinterpret the utility of novel agents for which outcomes are assessed based on minimal metastatic disease, imprecise quality-of-life assessments, or PSA-only measurements.[36] While randomization can overcome some of these problems, developing a context regarding the true impact of novel therapies requires consideration of the merits and biological impact of the endpoints that are employed. This issue can be mitigated by the use of well-defined trial designs, prespecified times of analysis, and the application of randomization to ensure that novel compounds truly offer survival benefit over established therapies.
Financial Disclosure:Dr. Raghavan sits on the President’s Strategy Council for Sanofi Aventis, is an ad hoc member of the FDA Oncology Drug Advisory Committee, and intermittently consults for Gerson Lehrman. Dr. Burgess owns Exelixis, Inc, stock valued at less than $10,000.
References:
REFERENCES
1. Goldenberg SL, Bruchovsky N, Gleave ME, et al. Intermittent androgen suppression in the treatment of prostate cancer: a preliminary report. Urology. 1995;45:839-44.
2. Calais da Silva FE, Bono AV, Whelan P, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the south european uroncological group. Eur Urol. 2009;55:1269-77.
3. Mottet N, Van Damme J, Loulidi S, et al. Intermittent hormonal therapy in the treatment of metastatic prostate cancer: a randomized trial. BJU Int. 2012;110:1262-9.
4. Salonen AJ, Taari K, Ala-Opas M, et al. The FinnProstate Study VII: intermittent versus continuous androgen deprivation in patients with advanced prostate cancer. J Urol. 2012;187:2074-81.
5. Hussain M, Tangen CM, Higano CS, et al. Intermittent (IAD) versus continuous androgen deprivation (CAD) in hormone sensitive metastatic prostate cancer (HSM1PC) patients (pts): results of S9346 (INT-0162), an international phase III trial. J Clin Oncol. 2012;30(suppl): abstr 4.
6. Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025-33.
7. Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563-71.
8. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
9. Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742-8.
10. Ryan CJ, Smith MR, De Bono JS, et al, on behalf of the COU-AA-302 investigators. Interim analysis (IA) results of COU-AA-302, a randomized, phase III study of abiraterone acetate (AA) in chemotherapy-naive patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2012;30(suppl): abstr LBA4518.
11. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787-90.
12. Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437-46.
13. De Bono JS, Fizazi K, Saad F, et al, for the AFFIRM Investigators. Primary, secondary, and quality-of-life endpoint results from the phase III affirm study of MDV3100, an androgen receptor signaling inhibitor. J Clin Oncol. 2012;30(suppl): abstr 4519.
14. Ileana E, Loriot Y, Albiges L, et al. Abiraterone in patients with metastatic castration-resistant prostate cancer progressing after docetaxel and MDV3100. J Clin Oncol. 2012;30(suppl): abstr 4554.
15. Rathkopf DE, Morris MJ, Danila DC, et al. A phase I study of the androgen signaling inhibitor ARN-509 in patients with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2012;30(suppl): abstr 4548.
16. Agus DB, Stadler WM, Shevrin DH, et al. Safety, efficacy, and pharmacodynamics of the investigational agent orteronel (TAK-700) in metastatic castration-resistant prostate cancer (mCRPC): updated data from a phase I/II study. J Clin Oncol. 2012;30(suppl 5): abstr 98.
17. Eisenberger MA, Kattan MW, Sternberg CN, et al. A multinational phase III adjuvant study of immediate (I) versus deferred (D) chemotherapy (C)/hormone therapy (HT) after radical prostatectomy (RP): TAX-3501. J Clin Oncol. 2012;30(suppl): abstr 4570.
18. Flaig TW, Tangen CM, Hussain MHA, et al. Randomization reveals unexpected acute leukemias in SWOG prostate cancer trial. J Clin Oncol. 2008;26:1532-6.
19. Scolieri MJ, Altman A, Resnick MI. Neoadjuvant hormonal ablative therapy before radical prostatectomy: a review. Is it indicated? J Urol. 2000;164:1465-72.
20. Kang TY, Nichols P, Skinner E, et al. Functional heterogeneity of prostatic intraepithelial neoplasia: the duration of hormonal therapy influences the response. BJU Int. 2007;99:1024-7.
21. Efstathiou E, Davis JW, Troncoso P, et al. Cytoreduction and androgen signaling modulation by abiraterone acetate (AA) plus leuprolide acetate (LHRHA) versus LHRHA in localized high-risk prostate cancer (pCa): preliminary results of a randomized preoperative study. J Clin Oncol. 2012;30(suppl): abstr 4556.
22. Taplin M-E, Montgomery RB, Logothetis C, et al. Effect of neoadjuvant abiraterone acetate (AA) plus leuprolide acetate (LHRHa) on PSA, pathological complete response (pCR), and near pCR in localized high-risk prostate cancer (LHRPC): results of a randomized phase II study. J Clin Oncol. 2012;30(suppl): abstr 4521.
23. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813-22.
24. Robinson RG, Preston DF, Schiefelbein M, et al. Strontium 89 therapy for the palliation of pain due to osseous metastases. JAMA. 1995;274:420-4.
25. Serafini AN, Houston SJ, Resche I, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16:1574-81.
26. Nilsson S, Franzen L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007;8:587-94.
27. Parker C, Nilsson S, Heinrich D, et al. Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA). J Clin Oncol. 2012;30(suppl): abstr LBA4512.
28. Pisters LL, Troncoso P, Zhau HE, et al. c-met proto-oncogene expression in benign and malignant human prostate tissues. J Urol. 1995;154:293-8.
29. Verras M, Lee J, Xue H, et al. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67:967-75.
30. Kim SJ, Johnson M, Koterba K, et al. Reduced c-Met expression by an adenovirus expressing a c-Met ribozyme inhibits tumorigenic growth and lymph node metastases of PC3-LN4 prostate tumor cells in an orthotopic nude mouse model. Clin Cancer Res. 2003;9:5161-70.
31. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel met and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298-2308.
32. Hussain M, Smith MR, Sweeney C, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): results from a phase II randomized discontinuation trial. J Clin Oncol. 2011;29(suppl): abstr 4516.
33. Smith MR, Sweeney C, Rathkopf DE, et al. Cabozantinib (XL184) in chemotherapy-pretreated metastatic castration resistant prostate cancer (mCRPC): results from a phase II nonrandomized expansion cohort (NRE). J Clin Oncol. 2012;30(suppl): abstr 4513.
34. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302-9.
35. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26:1148-59.
36. Raghavan D. Novel therapies for advanced prostate cancer: have we widened the goal posts too far? Ann Oncol. 2012;23:2473-5.
37. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22.
38. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147-54.
39. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513-20.