Combining Camptothecins with Radiation Might Produce Powerful Antitumor Regimen
NASHVILLE, Tennessee-"Camptothecins have radiosensitizing as well as cytotoxic effects, and combining a camptothecin such as irinotecan with radiation on the right schedule might produce a powerful antitumor regimen," according to Allan Chen, MD, PhD. Dr. Chen is assistant professor of radiation oncology at Vanderbilt University Medical Center in Nashville, Tennessee.
NASHVILLE, Tennessee"Camptothecins have radiosensitizing as well as cytotoxic effects, and combining a camptothecin such as irinotecan with radiation on the right schedule might produce a powerful antitumor regimen," according to Allan Chen, MD, PhD. Dr. Chen is assistant professor of radiation oncology at Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Chen reviewed previous phase I/II chemoradiation trials of irinotecan (Camptosar) for non-small-cell lung cancer (NSCLC). "The promising results of these early trials," he said, "indicate that there is an urgent need to speed up understanding of the cytotoxic interaction of radiation and the camptothecin drugs."
Combining chemotherapy with radiation presents a number of potential advantages. One of these, Dr. Chen noted, is spatial cooperation: chemotherapy offers systemic control, while radiation treats tumor sanctuary sites. A second potential benefit is that chemotherapy reduces tumor burden at the local primary tumor site. Some types of chemotherapy, including camptothecins, are also radiosensitizers and can increase the efficacy of a given dose of radiation.
Attractive Therapeutic Target
Dr. Chen said that topoisomerase I is attractive as a therapeutic target because it is elevated in both proliferating and quiescent tumor cells and because targeting topoisomerase I can have both cytotoxic and radiosensitizing effects. "Advances in studies of the molecular mechanism of action of topoisomerase I as a therapeutic target provide a unique opportunity for development of better cancer therapy," he said.
Cell killing by camptothecins requires active DNA synthesis. Dr. Chen explained that topoisomerase I forms a transient covalent linkage with the 3' end of the cleaved DNA during catalysis. Camptothecins trap this reversible topoisomerase I-DNA cleavable complex and convert a DNA topology-modifying activity into a DNA-breaking poison. This damages DNA through interaction with ongoing DNA replication.
Radiation sensitivity varies during the mitotic cell cycle. "Cells in G2-phase are the most sensitive to radiation. Those in S-phase are the most resistant. Combining an S-phase-specific cytotoxic drug with radiation theorectically should produce an additive effect," Dr. Chen said.
"Radiosensitization induced by camptothecins does not occur if radiation is given prior to camptothecin treatment, which suggests that it would not be effective to treat patients with radiation first followed by chemotherapy," Dr. Chen said. "Topoisomerase I-directed radiosensitization is schedule dependent and requires an intact stereospecific interaction between drug and topoisomerase I." Topoisomerase I mediated radiosensitization is also time dependent.
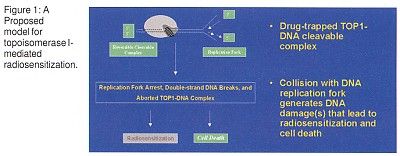
Proposed Explanatory Model
Dr. Chen proposed a model to explain topoisomerase I-mediated radiosensitization (Figure 1). "After the camptothecin-trapped topoisomerase I-DNA cleavable complex is formed, a collision with the DNA replication apparently generates DNA damage that leads to radiosensitization and cell death," he said. This process might be mediated by p53.
"Human cervical cancer HeLa cells are infected with the high-risk human papillomaviruses (HPVs) types 16 and 18 and contain the oncoproteins E6 and E7. The E6 protein encoded by the oncogenic HPVs targets p53 for ubiquitin-dependent proteolysis and produces a relatively suppressed p53 state in HeLa cells," Dr. Chen explained. "Campto-thecin produces little or no radiosensitization in such cells." Studies of the human colon cancer HCT-116 cell line compared to isogenic cells with p53 knocked out also support this idea.
The heterodimeric Ku protein also contributes to the theory. "The Ku protein binds to the ends of DNA breaks," Dr. Chen said. "These proteins, which are the regulatory subunits of DNA-dependent protein kinase, are involved in recombination and in nonhomologous end joining. Ku-deficient cells are hypersensitive to ionizing radiation and to topoisomerase II drugs." Ku-deficient cells are also less responsive to topoisomerase I-mediated radiosensitization.
Dr. Chen proposed that topoisomerase I radiosensitization might reduce the tumor cell’s ability to repair radiation-induced DNA damage that would be sublethal and repairable in cells not treated with camptothecins.
"Our findings suggest that DNA damage plays a key role in regulating topoisomerase I-mediated radiosensitization in mammalian cells," Dr. Chen stated. "With a better understanding of the mechanism of topoisomerase I-mediated radiosensitization, we might be able to develop novel radiation sensitizers and chemoradiation regimens for cancer therapy."