Rubitecan Appears Active in Advanced Pancreatic Cancer
NEW YORK-The camptothecin analog rubitecan (9-nitrocamptothecin) is safe and active in advanced pancreatic cancer, the combined experience from two phase II trials suggests.
NEW YORKThe camptothecin analog rubitecan (9-nitrocamptothecin) is safe and active in advanced pancreatic cancer, the combined experience from two phase II trials suggests.
"Rubitecan produces partial responses and substantial disease stabilization in heavily pretreated patients," said Kenneth A. Foon, MD, director of the Barrett Cancer Center, University of Cincinnati Medical School. "It is well tolerated, with manageable nausea, vomiting, and myelosuppression as the most common adverse events."
Rubitecan could fill a need for effective systemic therapy for pancreatic cancer, which results in about 29,000 US deaths annually. Five-year survival is about 5%, Dr. Foon said at the Chemotherapy Foundation Symposium XVIII.
In a phase I trial, rubitecan was active against pancreatic cancers, among other solid tumors, with myelosuppression being the dose-limiting toxicity. "Rubitecan has an excellent activity-to-toxicity ratio, and can be given orally," he said.
An initial phase II trial included 107 patients, most of whom were stage IV (73%) and had Zubrod performance status of 1 (78%). Fifty were previously treated; of those, 33 had failed gemcitabine (Gemzar). Rubitecan 1.5 mg/m2 daily was given orally, in weekly cycles of 5 days plus 2 days rest.
Median survival was 6.5 months for all 107 patients, 7.3 months for chemotherapy-naïve patients, and 4.7 months for those who failed gemcitabine. Nine patients (8.4%) had a response by standard objective criteria (tumor reduction greater than 50%), while stable disease was seen in 10 patients (9.4%).
Those single-center findings were followed by a multicenter phase II trial in 58 patients with refractory pancreatic cancer, 90% of whom had stage IV disease. Almost all (90%) had been previously treated with a gemcitabine-based regimen. The same dose of rubitecan was used. Doses could be adjusted up or down based on toxicity, and patients were treated until disease progressed.
Of 45 patients who had measurable disease, and thus were considered evaluable, 4 (9%) had a partial response and 11 (24%) had stable disease. Among the subset of evaluable patients who failed gemcitabine (n = 40), there was only one partial response. CA 19-9 response, among the 27 evaluable patients with abnormal Ca 19-9 values, included two complete responses and three partial responses, for a 20% overall response rate.
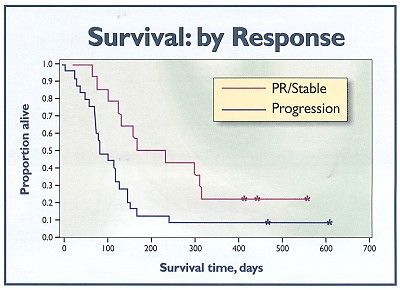
Survival by intent-to-treat analysis was a median of 86 days for all 58 patients, with 9% alive at 1 year. Patients who received at least 8 weeks of therapy had a median survival of 154 days, with 17% alive at 1 year. Among evaluable patients with either stable disease or a partial response, median survival was 301 days with 1-year survival of 36% (see Figure). For the four patients who had a partial response, median survival was 358 days, and half were still alive at 1 year.
Every patient experienced some degree of anemia (almost all had grade 1-2 anemia at baseline). Dr. Foon said the incidence of grade 3-4 adverse events was "quite acceptable," with myelosuppression being the most common toxicity. Cystitis, a major adverse event in the single-center trial, was only a "minor problem" in this study because patients were adequately orally hydrated. "Rubitecan was clearly well tolerated," he said, "and the multicenter study confirms the efficacy data from the single-center trial."