Irinotecan Results Continue to Be Encouraging in Colorectal Cancer
NASHVILLE, Tennessee-Encouraging results from phase II and III clinical trials of irinotecan (Camptosar) in colorectal cancer over the past several years as well as future directions for research were reviewed by Mace L. Rothenberg, MD. He is associate professor of medicine and Ingram Associate Professor of Cancer Research at Vanderbilt University Medical Center, Nashville.
NASHVILLE, TennesseeEncouraging results from phase II and III clinical trials of irinotecan (Camptosar) in colorectal cancer over the past several years as well as future directions for research were reviewed by Mace L. Rothenberg, MD. He is associate professor of medicine and Ingram Associate Professor of Cancer Research at Vanderbilt University Medical Center, Nashville.
Second-Line Therapy
Irinotecan had "consistent activity demonstrated in phase II trials, including response rates of 12% to 23%, median response duration of 5 to 7 months, and median survival of 8 to 11 months," Dr. Rothenberg reported.
Two single-agent phase III trials have been performed. The first, a European study of recurrent colorectal cancer after 1 to 2 prior regimens, compared irinotecan to best supportive care. Overall survival was significantly better with irinotecan than with best supportive care (Figure 1). Median survival was 9.2 months with irinotecan vs 6.5 months with best supportive care. In addition, quality of life (QOL) was improved with irinotecan treatment.
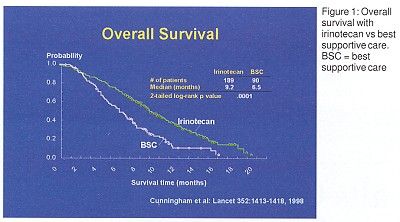
The second trial compared the same irinotecan regimen to infusional fluorouracil (5-FU) in recurrent colorectal cancer after a single prior regimen, and found QOL measures to be similar for both treatments.
First-Line Therapy
Single agent response rates of 18% to 32% and median survival of about 12 months inspired researchers to try combination therapy with irinotecan, fluorouracil (5-FU), and leucovorin in patients with stage IV colorectal cancer not previously treated for metastatic disease.
Combination Therapy Tried
A phase III trial of the Saltz regimen of irinotecan/5-FU/leucovorin in patients with stage IV colorectal cancer was conducted in the United States, Canada, Australia, and New Zealand. The first group in this study received irinotecan 125 mg/m2 each week for 4 weeks, repeated every 6 weeks. The second group received irinotecan125 mg/m2, leucovorin 20 mg/m2, 5-FU 500 mg/m2 weekly for 4 weeks, repeated every 6 weeks. The third group received leucovorin 20 mg/m2 and 5-FU 425 mg/m2 daily for 5 days, repeated every 4 weeks.
The three-drug regimen was superior to the two-drug regimen in all measures of efficacy. The response rate was 50% vs 28% (P < .001). Progression-free survival was 7.0 months vs 4.3 months (P = .004), and overall survival was 14.8 months vs 12.6 months (P = .04).
Subset analysis suggested that patients with performance status of 0 (asymptomatic) were the most likely to benefit from treatment, while those who had PS1 or PS2 had some improvement but no increase in overall survival. Patients with LDH within normal limits also gained more from irinotecan/5-FU/leucovorin than from 5-FU/leucovorin, or from irinotecan alone.
Another phase III study was conducted in Europe, Israel, and South Africa among previously untreated patients. Using the Douillard regimen, the first group of patients was randomized to irinotecan plus leucovorin 200 mg/m2 over 2 hours, followed by bolus 5-FU 400 mg/m2, followed by 5-FU 600 mg/m2 IV 22 hours on days 1 and 2, repeated every 2 weeks or irinotecan 80 mg/m2 plus leucovorin 500 mg/m2 over 2 hours, followed by 5-FU 2,300 mg/m2 as a 24-hour IV infusion weekly for 6 weeks, repeating every 7 weeks. Patients in the second group were randomized to these two regimens without irinotecan.
Patients on the irinotecan/5-FU/leucovorin arm fared better in all measures of efficacy. The response rate was 49% vs 31% (P < .001); progression-free survival was 6.7 months vs 4.4 months (P < .001); and overall survival was 17.4 months vs 14.1 months (P < .031).
Quality-of-Life Issues
"The irinotecan/5-FU/leucovorin regimen had no adverse impact on QOL," Dr. Rothenberg said. Toxicity, however, was "not insignificant," and addition of irinotecan was associated with a significant increase in grade 3-4 neutropenia.
"The addition of irinotecan to first-line chemotherapy for colorectal cancer improves survival and maintains QOL. Questions remain about whether the irinotecan must be given simultaneously with 5-FU/leucovorin or simply integrated into front-line chemotherapy," Dr. Rothenberg said. A phase II multicenter, open-label trial of alternating irinotecan and 5-FU/leucovorin, which was presented at the 36th annual meeting of the American Society of Clinical Oncology (ASCO), but is not yet published, showed median time to tumor progression of 6.9 months, median survival of 17.8 months, and 1-year estimated survival of 51% with the alternating regimen.
Important Questions Remain
"An important question at present is whether all patients with metastatic colorectal cancer should receive irinotecan/5-FU/leucovorin as first-line therapy," Dr. Rothenberg said. "Between May of 2000 and February of 2001, utilization of irinotecan/5-FU/leucovorin as first-line treatment increased from 35% to 70%. Reasons for nonusage included poor performance status, high LDH, liver dysfunction, poor tolerance for diarrhea, desire for oral therapy, or participation in clinical trials."
Investigators are also asking whether irinotecan could be given with regimens other than 5-FU/leucovorin, most commonly oxaliplatin. "Irinotecan and oxaliplatin have shown sequence-dependent synergy in vitro against HT29 colorectal cancer cells," Dr. Rothenberg said. This combination is being investigated in North Central Cancer Treatment Group/Intergroup study N-9741.
"We also need to know whether irinotecan can improve survival and increase cure rates in patients with nonmetastatic, locally advanced colorectal cancer," Dr. Rothenberg said. This is being investigated in CALGB trial C-89803 and in several European studies in stage II/III colorectal cancer.
"Should irinotecan/5-FU/leucovorin be used as standard therapy for patients with high risk stage II or III colorectal cancer? This issue is controversial. Dr. Rothenberg argues that the answer to that question is no. "Irinotecan causes chromosomal damage and there is the potential for an increased rate of secondary malignancies. Irinotecan adds toxicity to 5-FU/leucovorin, and there is as yet no evidence that this is clearly worthwhile," Dr. Rothenberg explained.
A number of symposium participants expressed opinions differing with Dr. Rothenberg regarding its use as adjuvant therapy. They protested that they would personally be willing to undertake the risks of the regimen in return for its potential benefits.
Looking back at previous studies and ahead to future research, Dr. Rothenberg concluded: ‘‘Over the past 10 years, irinotecan has established itself as an important element in the treatment of patients with advanced colon cancer. Now our challenge is to further explore the best ways and optimal settings to use irinotecan in patients with all stages of the disease.’’