Commentary (Kaklamani/O’Regan): Aromatase Inhibitors as Adjuvant Therapy in Breast Cancer
The use of aromatase inhibitorshas increased dramatically inthe past few years as a resultof the emergence of new, more specificagents, such as anastrozole(Arimidex), exemestane (Aromasin),and letrozole (Femara). This class ofagents effectively blocks the peripheralformation of estradiol, decreasingits concentration to less than 10%,while maintaining selectivity.[1]Evaluation of these selective aromataseinhibitors as adjuvant therapyfor early-stage breast cancer wasbased on the findings of trials inmetastatic breast cancer, summarizedby Visvanathan and Davidson, thatdemonstrated the equivalence and,in some cases, superiority of thearomatase inhibitors comparedwith megestrol and tamoxifen,including their superior side-effectprofile.[2-4]
The use of aromatase inhibitors has increased dramatically in the past few years as a result of the emergence of new, more specific agents, such as anastrozole (Arimidex), exemestane (Aromasin), and letrozole (Femara). This class of agents effectively blocks the peripheral formation of estradiol, decreasing its concentration to less than 10%, while maintaining selectivity.[1] Evaluation of these selective aromatase inhibitors as adjuvant therapy for early-stage breast cancer was based on the findings of trials in metastatic breast cancer, summarized by Visvanathan and Davidson, that demonstrated the equivalence and, in some cases, superiority of the aromatase inhibitors compared with megestrol and tamoxifen, including their superior side-effect profile.[2-4]
Although a number of trials are comparing aromatase inhibitors to tamoxifen in early-stage breast cancer (outlined in Table 2 of Visvanathan and Davidson's review), the only results available to date are from the Arimidex, Tamoxifen Alone or in Combination (ATAC) trial. Preliminary results of this trial, at a follow- up of 33 months, were presented at the San Antonio Breast Cancer Symposium in 2001.[5] Anastrozole was found to be superior to tamoxifen and the combination with a relative disease-free survival of 17%, which translates into an absolute benefit of 2%.[5] In addition, anastrozole reduced the risk of contralateral breast cancer by 58%.[5]
FIGURE 1
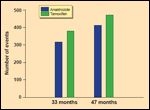
Preliminary and Longer-Term Follow-Up
Updated results of the ATAC trial were recently presented, at a follow- up of 47 months.[6] Disease-free survival remained significantly improved in the anastrozole arm, compared to the tamoxifen arm, with an absolute benefit of 2.3% in the overall population and 2.9% in the receptorpositive population (Figure 1).[6] However, the rate of contralateral breast cancer was no longer significantly different between anastrozole- and tamoxifen-treated patients (Figure 2).[6] Despite these findings of improved efficacy with the use of anastrozole, several important issues remain, as outlined by the authors in their review.
Superior Side-Effect Profile?
FIGURE 2
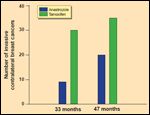
Contralateral Breast Cancers
The side-effect profile in the ATAC trial favored the use of anastrozole, which led to fewer treatment withdrawals.[7] Patients who received anastrozole experienced fewer hot flashes and less weight gain, compared to tamoxifen-treated patients.[7] More importantly, the estrogenic effect on the endometrium was weaker in the anastrozole arm, with significantly fewer cases of endometrial cancers.[ 8] Additionally, fewer thromboembolic and cerebrovascular events occurred in the anastrozole arm.[7]
However, important concerns linger about the effect of long-term estrogen deprivation produced by the aromatase inhibitors. Among these concerns are the increased fracture rate and effects on bone mineral den- sity noted in anastrozole-treated ATAC patients.[7] Although no firm guidelines have been delineated for the management of bone mineral density in patients taking anastrozole, recent data suggest that bisphosphonates can prevent the bone loss associated with the drug.
An Austrian trial demonstrated that there was no significant decrease in T score at 18 months in premenopausal patients treated with goserelin (Zoladex) and anastrozole when they were given zoledronate (Zabel, Zometa) every 6 months.[9] Additionally, exemestane prevents ovariectomy- induced bone loss in rats, suggesting that it may not produce the same degree of bone loss noted with anastrozole.[10]
Another important concern regarding the estrogen deprivation associated with anastrozole is the possible long-term effect on cognitive function, and as suggested by Visvanathan and Davidson, there may be other as yet unrecognized side effects associated with the use of aromatase inhibitors.
Which Patients Benefit From Aromatase Inhibitors?
The large number of patients accrued to the ATAC trial allowed several subset analyses to be performed. One of the more intriguing findings was that anastrozole is significantly better than tamoxifen only in patients with axillary node-negative breast cancer, with a trend toward improvement in patients with one to three positive lymph nodes but no benefit (vs tamoxifen) in patients with four or more positive lymph nodes.[6] However, in patients with node-positive breast cancer, disease-free survival was similar in the two treatment arms.[6]
In addition, in the 20% of patients who received adjuvant chemotherapy, no difference in disease- free survival was noted between patients treated with anastrozole and those treated with tamoxifen.[6] The lack of benefit for anastrozole over tamoxifen in patients who received chemotherapy may relate to the added benefit these patients received from adjuvant chemotherapy. Regardless,this finding does not completely explain the lack of benefit for anastrozole compared to tamoxifen in node-positive patients, because 30% of patients had positive lymph nodes but only 20% received chemotherapy.[5,7]
On the basis of these subgroup analyses, anastrozole can be considered superior to tamoxifen for the treatment of early-stage, axillary node-negative breast cancer. However, patients in this category have the best prognosis and are more likely to die from other causes. Because anastrozole will reduce bone mineral density and may increase the incidence of hip fractures-a significant cause of morbidity and mortality in older women-caution must be exercised when prescribing aromatase inhibitors for postmenopausal patients with axillary node-negative breast cancer.
Impact of HER2/neu
Although controversial, preclinical[ 11] and clinical data[12] suggest that hormone-receptor-positive, HER2/neu-positive breast tumors may be resistant to tamoxifen. As outlined by the authors, a neoadjuvant trial demonstrated that, compared with tamoxifen, the nonsteroidal aromatase inhibitor letrozole produces a superior clinical response rate and rate of breast-conserving surgery in patients with estrogen-receptor- positive tumors that overexpress HER2/neu and/or the epidermal growth factor receptor.[13]
To date, there are no data addressing the HER2/neu status of the tumors in the ATAC trial. It is possible that the benefit of anastrozole over tamoxifen in the ATAC trial may be due, in part, to an improved outcome in the patients with HER2/neu-positive tumors. Until further data are available, it would seem reasonable to recommend aromatase inhibitors to patients with HER2/neu-positive, early-stage breast cancer.
Conclusions
So why don't we implement treatment with aromatase inhibitors for all patients in the adjuvant setting? First, follow-up in the ATAC trial is still too short for drawing final conclusions about survival. Second, tamoxifen has been shown to have continued benefits even after therapy is stopped; the duration of therapy in the ATAC trial is still too short to make such observations about aromatase inhibitors.
Third, a subgroup analysis of the ATAC trial showed that the benefit with anastrozole over tamoxifen only applied to patients with node-negative breast cancer, and that the drugs were equally efficacious in node-positive breast cancer. Fourth, we have not seen ATAC data on the HER2/neu status of the patients enrolled. The benefit observed with anastrozole may be limited to individuals whose tumors are HER2/neu positive. Last, and most important, the long-term effects of estrogen deprivation produced by aromatase inhibitors remain a concern.
So what should be we looking for in the future? A longer follow-up of the ATAC trial as well as the studies currently under way may confirm the benefit of aromatase inhibitors in the adjuvant setting. They will also provide long-term data on the side effects of such therapy and, perhaps, identify populations that might benefit more from the use of aromatase inhibitors.
Based on available data, it would seem reasonable to recommend aromatase inhibitors for specific subsets of patients, including those with contraindications to tamoxifen, those with HER2/neu-positive tumors, and those who develop breast cancer while on selective estrogen-receptor modulator (SERM) therapy. Longer follow-up of the ATAC trial and the results of ongoing trials should define the role of aromatase inhibitors in early-stage breast cancer, in ductal carcinoma in situ, and as chemopreventive agents.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Goss PE, Strasser K: Aromatase inhibitorsin the treatment and prevention of breastcancer. J Clin Oncol 19:881-894, 2001.
2.
Buzdar A, Jonat W, Howell A, et al: Anastrozole,a potent and selective aromatase inhibitor,versus megestrol acetate in postmenopausalwomen with advanced breast cancer: Resultsof overview analysis of two phase III trials.Arimidex Study Group. J Clin Oncol 14:2000-2011, 1996.
3.
Buzdar A, Douma J, Davidson N, et al:Phase III, multicenter, double-blind, randomizedstudy of letrozole, an aromatase inhibitor,for advanced breast cancer versus megestrolacetate. J Clin Oncol 19:3357-3366, 2001.
4.
Kaufmann M, Bajetta E, Dirix LY, et al:Exemestane is superior to megestrol acetateafter tamoxifen failure in postmenopausal womenwith advanced breast cancer: results of aphase III randomized double-blind trial. TheExemestane Study Group. J Clin Oncol18:1399-1411, 2000.
5.
Abstracts of the 24th Annual San AntonioBreast Cancer Symposium: The ATAC (Arimidex,Tamoxifen, Alone or in Combination)adjuvant breast cancer trial in postmenopausalwomen. Breast Cancer Res Treat 69:210, 2001.
6.
The ATAC (“Arimidex,” Tamoxifen,Alone or in Combination) trial in postmenopausalwomen with early breast cancer â updatedefficacy results based on a medianfollow-up of 47 months. Breast Cancer ResTreat 76(suppl 1):12, 2002.
7.
Anastrozole alone or in combination withtamoxifen versus tamoxifen alone for adjuvanttreatment of postmenopausal women withearly breast cancer: First results of the ATACrandomised trial. Lancet 359:2131-2139,2002.
8.
Duffy SRG, Jackson TL, on behalf of theATAC Trialists Group: The ATAC early breastcancer trial in postmenopausal patients: Endometrialsub-protocol results (abstract 158).Proc Am Soc Clin Oncol 21:40a, 2002.
9.
Gnant M, Hausmaninger H, Samonigg H,et al. Changes in bone mineral density causedby anastrozole or tamoxifen in combinationwith goserelin (+/- zoledronate) as adjuvanttreatment for hormone receptor-positive premenopausalbreast cancer: results of a randomizedmulticenter trial (abstract 12). Programand abstracts of the 25th Annual San AntonioBreast Cancer Symposium; San Antonio, Tex;December 11-14, 2002.
10.
Goss P, Grynpas M, Qi S, et al: Theeffects of exemestane on bone and lipids in theovariectomized rat (abstract). Breast CancerRes Treat 69:224, 2001.
11.
Benz CC, Scott GK, Sorup JC, et al:Estrogen-dependent, tamoxifen-resistant tumorigenicgrowth of MCF-7 cells transfected withHER2/neu. Breast Cancer Res Treat 24:85-95,1993.
12.
De Laurentis M, Bianco A, Placido S: Ameta-analysis of the interaction between HER2expression and response to endocrine treatmentin advanced breast cancer. Biol Treat BreastCancer 2:11-14, 2002.
13.
Ellis MJ, Coop A, Singh B, et al: Letrozoleis more effective neoadjuvant endocrinetherapy than tamoxifen for ErbB-1- and/orErbB-2-positive, estrogen receptor-positive primarybreast cancer: Evidence from a phase IIIrandomized trial. J Clin Oncol 19:3808-3816,2001.