Considerations for the Management of Oncology Patients During the COVID-19 Pandemic
ABSTRACT Worldwide incidence and mortality due to the coronavirus disease 2019 (COVID-19) pandemic is greatest in the United States, with the initial epicenter in New York. In Nassau County, New York, where we practice, our institution has had more than 2500 cases and has discharged from the hospital more than 1000 patients. As many academic and private institutions have swiftly shifted their clinical and research priorities to address the pandemic, data are emerging regarding both the impact of malignancy on COVID-19 outcomes as well as the challenges faced in assuring that cancer care remains unimpeded. Of concern, recent studies of cancer patients primarily in China and Italy have suggested that advanced malignancy is associated with increased susceptibility to severe COVID-19 infection. At present, more than 500 clinical trials are underway investigating the pathogenesis and treatment of COVID-19, including expanded use of oncology drugs, such as small molecular inhibitors of cytokine pathways. Here, we begin by reviewing the latest understanding of COVID-19 pathophysiology and then focus our attention on the impact of this virus on hematologic and oncologic practice. Finally, we highlight ongoing investigational treatment approaches that are so relevant to the care of oncology patients during this extraordinary pandemic.
Huang is a hematology and oncology fellow at NYU Winthrop Hospital.

Rohatgi is a hematology and oncology fellow at NYU Winthrop Hospital.

Introduction
The 2019 novel coronavirus severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) is a single-stranded RNA virus that was first reported in Wuhan, China, in December 2019,1 and it is responsible for coronavirus disease 2019 (COVID-19). COVID-19 is highly contagious, spreading by droplets, respiratory secretions, and direct contact. Its symptoms can include bilateral interstitial pneumonia, acute respiratory distress syndrome (ARDS), septic shock, and, ultimately, multiorgan failure.2,3 The virus has since rapidly spread to more than 100 countries and was declared a pandemic on March 11, 2020, by the World Health Organization.4
As of September 9, 2020, there were 27,699,974 confirmed cases of COVID-19 with 18,636,426 recovered and 900,239 deaths worldwide. In the United States, there were 6,358,983 confirmed COVID-19 cases; New York was a major epicenter, reporting 441,154 cases and 33,013 deaths.5 The numbers of new cases are still increasing across the United States, but they have declined dramatically in New York, likely consequent to the adoption of social distancing measures.
While many therapeutic strategies are currently being evaluated as possible COVID-19 treatments, there are currently no highly effective antiviral therapies or vaccines available to combat this virus, and mortality in severe disease remains high.6 Risk factors for severe illness resulting from COVID-19 are age greater than 65 years, diabetes, chronic lung disease, and obesity,7 and cancer patients who contract COVID-19, in particular, have been shown to have worse outcomes.8
Schneider is chief, Division of Oncology-Hematology and director, Thoracic Lung Cancer Program and NYU Winthrop Hospital.

Braunstein is assistant professor of medicine, program director Hematology/Oncology Fellowship, NYU Long Island School of Medicine and NYU Winthrop Hospital.

In the initial experience reported from Wuhan, China, 1% of cancer patients were noted to contract COVID-19, compared with just 0.29% incidence in the general population.8 This may be attributed to greater detection rates in more closely surveilled cancer patients, but it could also be associated with nosocomial exposures and diminished immune defenses.8 Cancer patients were also observed to be at higher risk for the development of severe COVID-19, which may be due to generally advanced age, increased prevalence of tobacco use, and higher incidence of comorbid pulmonary disease.9 Liang et al also demonstrated that cancer patients were more likely to require intensive care or experience mortality as compared with other COVID-19 patients (39% vs 8%).9 Cancer treatment may also increase COVID-19 susceptibility. In a small subset of patients reported by the National Health Commissions of the People’s Republic of China, those cancer patients who had undergone recent chemotherapy or surgery were observed to have a 75% likelihood of developing COVID-19 illness as compared with a 43% incidence in other cancer patients.9 However, this study by Liang et al included only 18 patients (1%) of 1590 COVID-19 cases had a history of cancer, making it difficult to make meaningful conclusions based on the small proportion of cancer patients included. Other studies, while also underpowered, have similarly suggested that patients with cancer are at greater risk for severe COVID-19.10
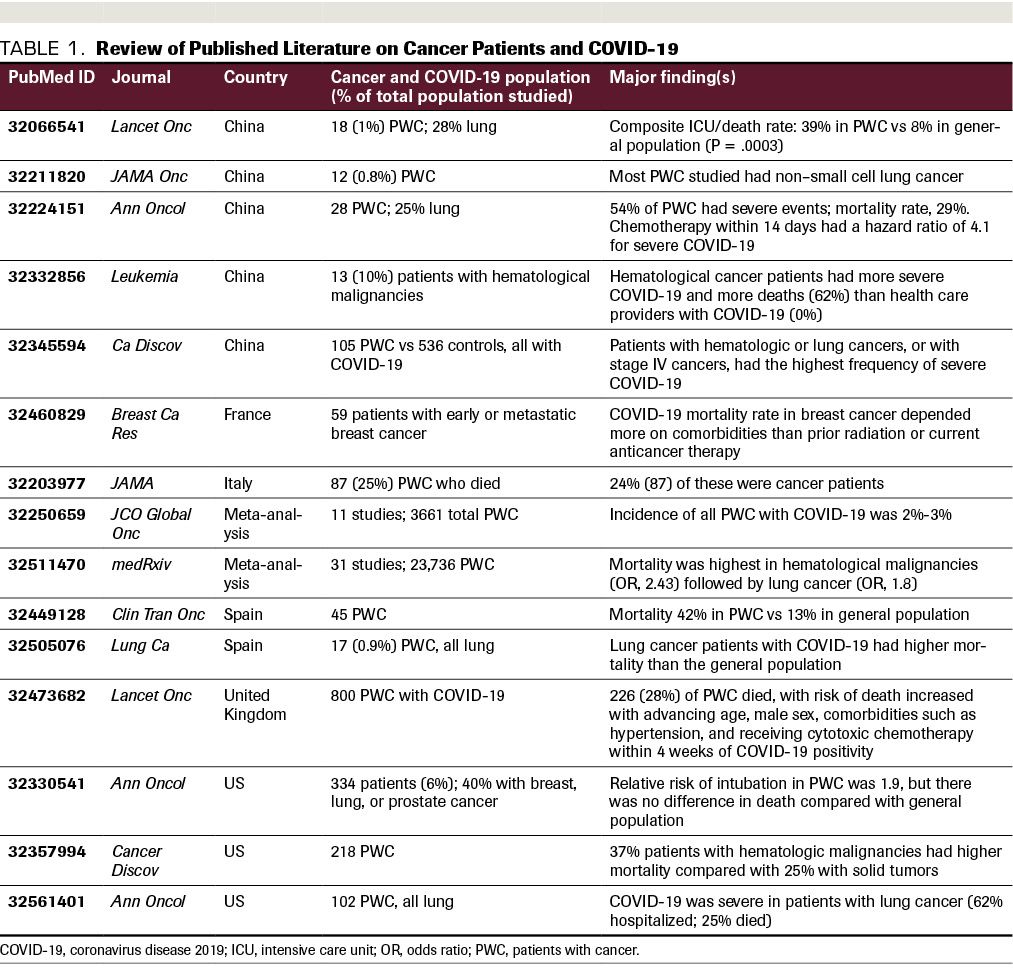
Patients with lung cancer and those with hematologic malignancies appear to be most vulnerable to COVID-19, presumably due to diminished pulmonary reserve in the former and immunodeficiency in the latter.11 In a report of 28 patients with cancer infected with SARS-CoV-2 in 3 Wuhan hospitals, 7 (25%) were noted to have lung cancer, 4 (14%) had esophageal cancer, and 3 (10.7%) had breast cancer.10 Severe hypoxia and devastating viral infection in lung cancer patients has been attributed to compromised baseline pulmonary function. Patients with stage IV cancers were also noted to experience severe COVID-19 infection (70%) more often than earlier-stage patients (44%).10 While cancer patients with COVID-19 infection often presented with typical symptoms of fever, dry cough, fatigue, and dyspnea, they were also noted to have atypical presentations, notably including anemia and hypoproteinemia. This may be secondary to nutritional deficiency associated with their cancer, which affects their immunocompetency to combat respiratory pathogens.10 As summarized in Table 1, available data suggest that cancer patients have poorer outcomes from COVID-19 illness, prompting the publication of specific guidelines on how to manage cancer patients during this crisis.11,12
TABLE 1. Review of Published Literature on Cancer Patients and COVID-19
COVID-19 has also been associated with increased incidence of thromboembolic disease attributed to clinical hypercoagulability. For example, a study conducted in Tongji Hospital in Wuhan, China, evaluated 183 patients from January to February 2020 and showed that of the 21 who died from COVID-19, 15 (71.4%) met criteria for overt disseminated intravascular coagulation (DIC) (≥5 points based on the International Society on Thrombosis and Hemostasis criteria),13 had significantly higher D-dimer and fibrinogen degradation product levels, and longer prothrombin times on admission, compared with survivors.14 Another study at Union Hospital in Wuhan reported a 25% incidence of lower extremity deep venous thrombosis in patients with severe COVID-19 pneumonia in ICU, with high (40%) subsequent mortality. This same report also suggested that since COVID-19 can decrease lymphocyte count, this may lead to immune dysfunction and a higher risk of venous thromboembolism due to poor immunity and DIC.15 Hypercoagulability in COVID-19 may also be due to the dysfunction of endothelial cells caused by infection that promotes thrombin formation and inhibits fibrinolysis.13 Some patients who died from COVID-19 were reported to have ischemic changes, with ecchymosis of their fingers and toes due to hypercoagulability from DIC and the development of microthrombi.16
Li et al proposed that early anticoagulation with low-molecular-weight heparin (LMWH) and intravenous immunoglobulins (Igs) would block the clotting formation seen in DIC and inhibit cytokine release storm (CRS), respectively.16 They recommend empiric anticoagulation for patients with D-dimers greater than 4 times the upper limit of normal range in the absence of contraindications. Lin et al recommend LMWH at 100 U/kg every 12 hours for at least 3 to 5 days.8 In addition, a study performed in Tongji Hospital in Wuhan reviewed 449 patients with severe COVID-19, with 94 patients receiving LMWH (40-60 mg/day) and 5 receiving unfractionated heparin (1000-15,000 U/day) for 7 days or longer; the results showed that the 28-day mortality for LMWH or unfractionated heparin users with a sepsis-induced coagulopathy score of ≥4 and D-dimers >6 times the upper limit of normal was lower than that of non-
LMWH or unfractionated heparinusers.14 This has led several institutions, including our own, to institute anticoagulation protocols based on various parameters such as D-dimer levels.17
Pathophysiology of COVID-19
Coronaviruses are large, single-stranded, positive-sense RNA strand that encapsulate within a membrane envelope surrounded by glycoprotein spikes, forming a crown-like appearance.18 Less-pathogenic endemic human coronaviruses such as OC43, HKU1, NL63, and 229E exist, causing seasonally, self-limited upper respiratory symptoms.19 In contrast, more severe respiratory symptoms are caused by zoonotic human coronaviruses, including severe acute respiratory distress syndrome coronavirus (SARS-CoV) discovered in November 2002 in Guangdong, China; Middle Eastern respiratory syndrome-related coronavirus (MERS-CoV) identified in 2012 in Saudi Arabia; and COVID-19.18 The subfamily, Coronavirinae, is divided into 4 classes of coronaviruses: α, β, δ, and γ. Sequencing data from SARS-CoV-2 share high degrees of homology—87.9% and 87.2%—with β coronavirus from Chinese bats, bat-SL-CoVZC45 and bat-SL-CoVZXC21, respectively.18 In comparison, SARS-CoV-2 is less genetically similar to its zoonotic human coronavirus counterparts, with 79% homologous to SARS-CoV and 50% homologous to MERS-CoV.18
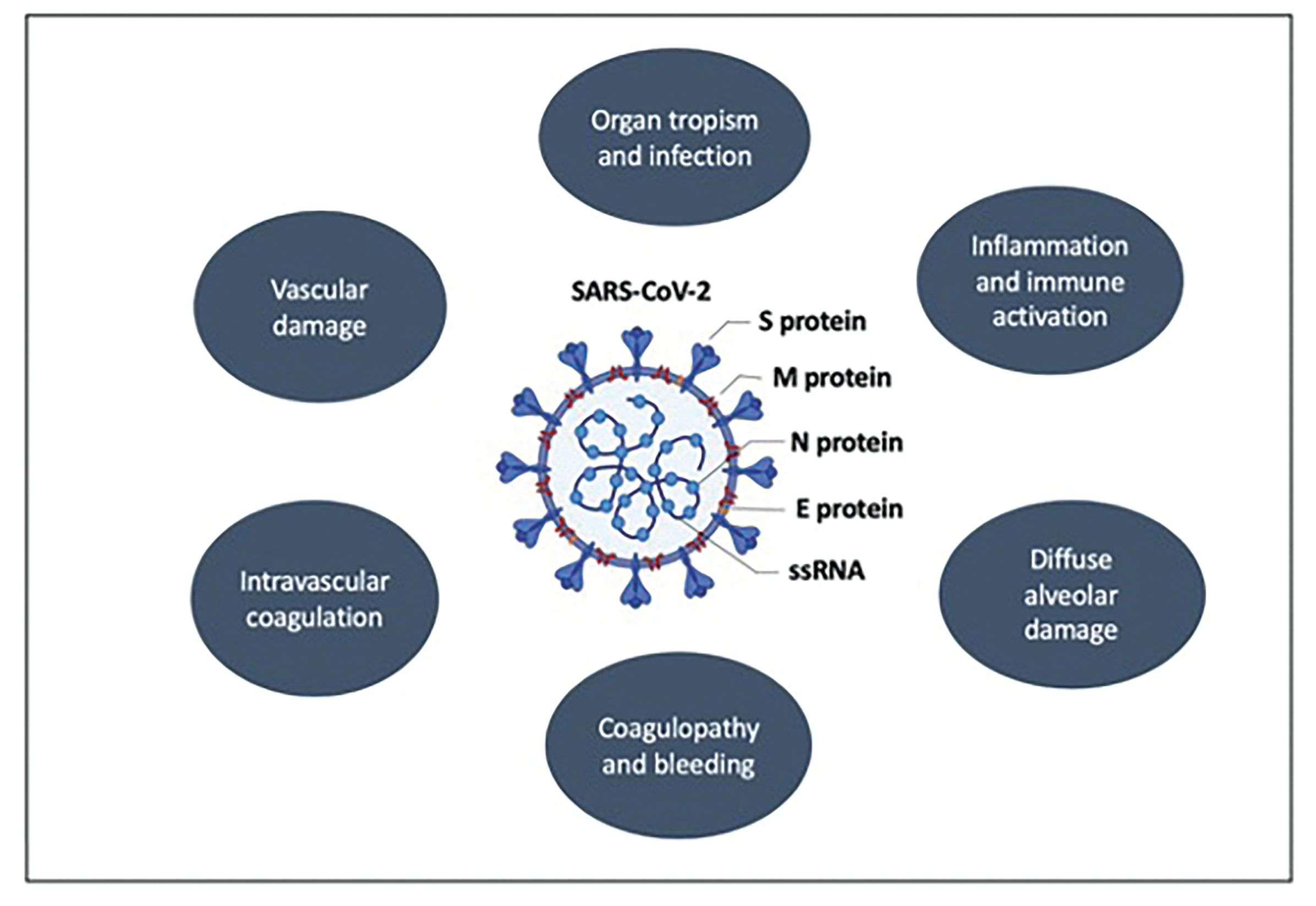
SARS-CoV and SARS-CoV-2 contain structural proteins, including glycosylated spike (S) protein, that targets angiotensin-converting-enzyme 2 (ACE2) on the surface of host cells. Upon entry into the membrane, the viral genome is released as a single-stranded, positive-sense RNA and is translated into viral polyproteins (pp1a and pp1ab) and cleaved by effector proteins, (viral proteinases 3CLpro and PLpro).2,18 Type II pneumocytes and the apical surface of ciliated airway epithelial cells within lungs express ACE2, which is a negative regulator in the renin-angiotensin system. ACE2 has been shown to promote anti-inflammatory and antifibrotic effects, and it protects these cells from ARDS.19 In SARS-CoV-2, ACE2 ectodomain can shed as a result of spikes from the viral glycoprotein, reducing the catalytic function of ACE2 and promoting ARDS.19 In addition, SARS-CoV-2 has been shown to reduce synthesis of interferon-α and interferon-β and to increase inflammatory cytokines and chemokines.20 In lung adenocarcinomas, ACE2 gene expression has been higher in malignant as compared with normal lung tissue, especially in the proximal proliferative subtype. On the other hand, most squamous lung cancers exhibit similar ACE2 expression in both normal and malignant cells. One exception is the primitive subtype of squamous cell lung cancer, in which ACE2 expression has been shown to be higher in normal cells as compared with their adjacent malignant counterpart cells, which has been associated with increased susceptibility to SARS-CoV-2 infection in this particular subpopulation.21 A summary of the pathophysiologic mechanisms of SARS-CoV-2 is shown in Figure 1.
Figure 1. Summary of Pathophysiologic Mechanisms of SARS-CoV-2 . Viral components involved in pathogenesis of coronavirus disease 2019 include the spike proteins (S) S1 and S2, which adhere to the ACE2 receptor; the matrix protein (M), which allows for bud release; and nucleocapside (N) and envelope (E), which surround the viral single-stranded RNA (ssRNA) genome and modulate immune evasion.
Investigational Agents for COVID-19
Treatment approaches for COVID-19 are continually evolving and there is currently no consensus therapeutic approach. Severe infection in COVID-19 is characterized by a CRS, rich in highly proinflammatory cytokines such as interleukin (IL)-1 and IL-6. Critically ill patients infected with COVID‐19 often suffer from multiorgan dysfunction, including respiratory failure, renal failure, and eventually cardiovascular collapse, leading to death. Oncology patients are especially vulnerable to this disease due to their overall frailty and immunocompromised state. A large number of antiviral therapies, including remdesivir, are under investigation, and as described below, several oncology drugs are also being repurposed to target COVID-19.
Anticytokine strategies. Because critically ill patients may be most challenged by endogenous cytokine release, even after viral eradication a large focus of attention has been focused upon the use of an agent to directly neutralize the CRS. Tocilizumab, an anti–IL-6 monoclonal antibody used to manage CRS associated with chimeric antigen receptor T-cell therapy, has been studied to suppress the toxic proinflammatory response in COVID-19. IL-6 may be a particularly important target, because increased IL-6 levels are released from the injured pulmonary bronchial and alveolar epithelial cells seen in ARDS22 and IL-6 levels were directly associated with the severity of SARS infections. Other cytokines—including IL-2, IL-6, IL-7, IL-10, granulocyte colony stimulating factor, 10 kD interferon γ-induced protein, monocyte chemoattractant protein-1, macrophage inflammatory protein 1-α (MIP-1α), and tumor necrosis factor (TNF)-α—have all been observed at elevated levels in COVID-19 patients requiring intensive care support.2,3 This suggests that steroids or other broad spectrum anti-inflammatory agents may play an important role in the management of catastrophic COVID-19 illness.
Convalescent plasma. Convalescent plasma is theorized to transfer neutralizing antiviral antibodies from patients who have recovered from COVID-19 infection to those who have been more recently infected.23 Unlike anticytokine strategies, convalescent plasma and other purely antiviral therapies must be given early in the course of COVID-19 illness, and certainly prior to viral eradication. The early experience with convalescent plasma at the Huoshenshan Hospital in Wuhan reported clinical and radiographic improvement in all 6 selected cases. However, convalescent plasma was administered late in the disease course of all these patients; prior to treatment, 4 of 6 had tested negative for residual virus and 5 of 5 had already tested positive for their own endogenous antiviral antibodies. The authors hypothesized that plasma might promote anti-inflammatory properties to prevent CRS,23 but it is also possible that these patients might have recovered just as well without plasma therapy. Similarly, Shen et al reported clinical improvement in 5 critically ill COVID-19 patients on ventilators in Shenzhen Third People’s Hospital, including extubation and ultimate discharge in 3 of these patients. But again, none of these patients were treated in the first 14 days after viral infection, which is when the introduction of antiviral antibodies is predicted to be most strategic.24 Most recently, the results of a small randomized clinical trial of convalescent plasma versus standard management failed to demonstrate protocol-defined clinical improvement or overall survival improvement at 28 days, but the trial was underpowered, including <10% patients with disease diagnosed within 14 days of randomization. It did demonstrate significantly enhanced viral clearance (90% vs 41% at 72 hours) in the subset of patients who tested positive for active virus at study entry.3
TABLE 2. Cancer-Directed Therapies Observed in the Past to Be Efficacious Against SARS-CoV and MERS-CoV
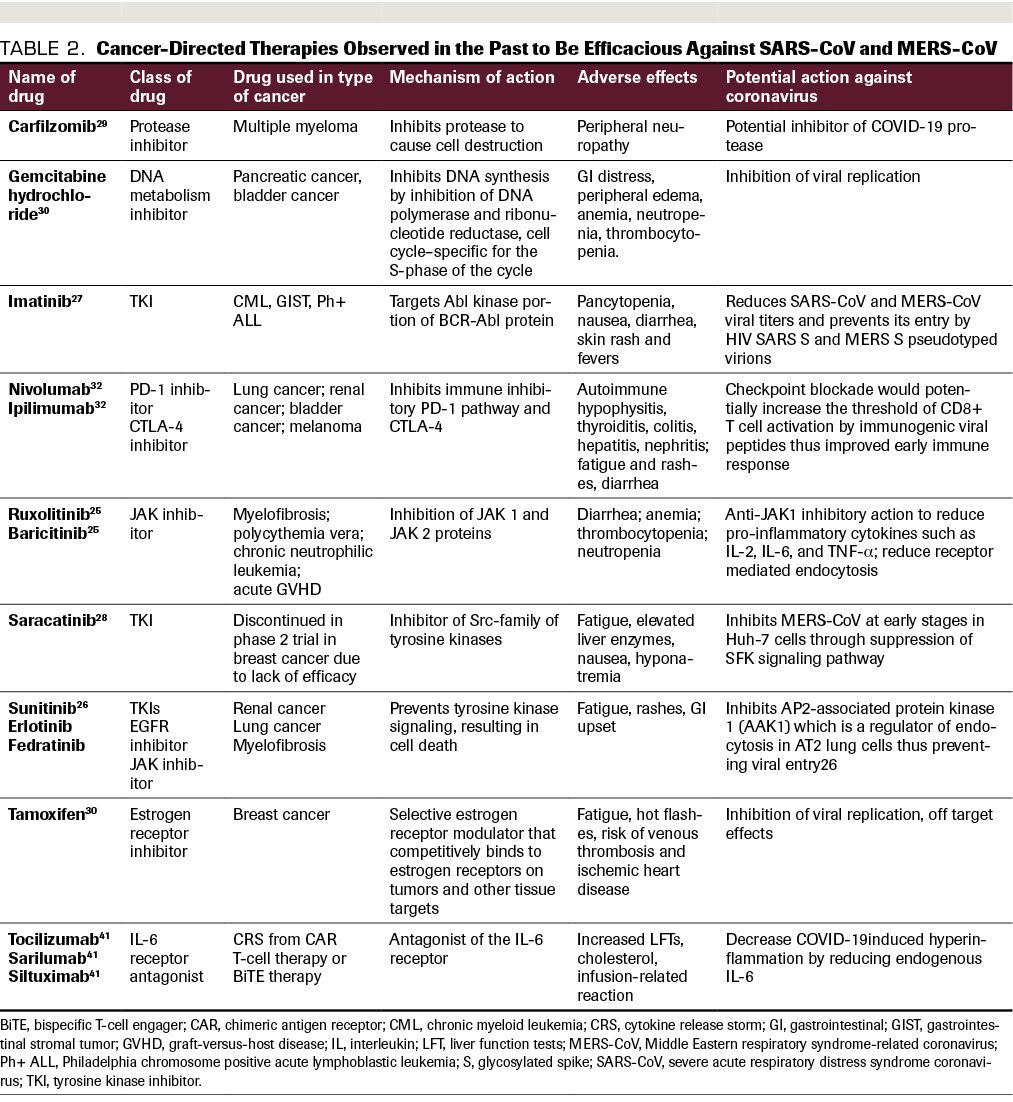
Repurposed antineoplastic agents. Many medications commonly used to treat cancer or cancer therapy–related adverse effects have also been explored for their potential beneficial effects in the treatment of SARS-CoV and MERS-CoV infections. Table 2 includes commonly used targeted oncology therapies which have mechanisms of actions as well as prior evidence of possible effectiveness in viral inhibition. Ruxolitinib, a JAK1/2 inhibitor, is associated with a decrease in proinflammatory cytokines downstream of JAK1/2 signaling and thereby leads to improved symptoms and quality of life in patients with myelofibrosis. Ruxolitinib also modulates the activity of natural killer cells, dendritic cells, T helper cells, and regulatory T cells, and was shown to be involved in the signaling pathway of proinflammatory cytokines such as IL-2, IL-6, and TNF-α.25 By reducing proinflammatory cytokines, ruxolitinib could be effective in decreasing the CRS in COVID-19 and is being evaluated in clinical trials. Tyrosine kinase inhibitors (TKIs) such as sunitinib, erlotinib, and fedratinib have been found as targets of the AAK1 protein, which is responsible for receptor-mediated endocytosis, the mechanism by which COVID-19 enters lung AT2 cells.26 Imatinib, another TKI, reduces SARS-CoV and MERS-CoV viral titers and prevents cellular entry by HIV SARS S and MERS S pseudotyped virions.27 Saracatinib, a TKI that was discontinued in a phase 2 study in breast cancer, has been observed to inhibit MERS-CoV at early stages in Huh-7 cells, via suppression of the SFK signaling pathway.28 Carfilzomib, a protease inhibitor commonly used to treat multiple myeloma, is being evaluated in COVID-19 illness for its potential ability to inhibit viral proteases.29
Estrogen receptor modulators such as tamoxifen represent another class of FDA-approved anticancer drugs that have shown potential activity in both MERS and SARS-CoV. Tamoxifen was identified in a drug screen to have significant antiviral activity; this is likely secondary to off-target effects, as the estrogen signaling pathway doesn’t play a role in viral entry. Gemcitabine hydrochloride is a deoxycytidine analogue that inhibits DNA synthesis and repair, and it was found in a drug screen to inhibit both MERS-CoV and SARS-CoV, although the mechanism is unclear as these are RNA viruses.30
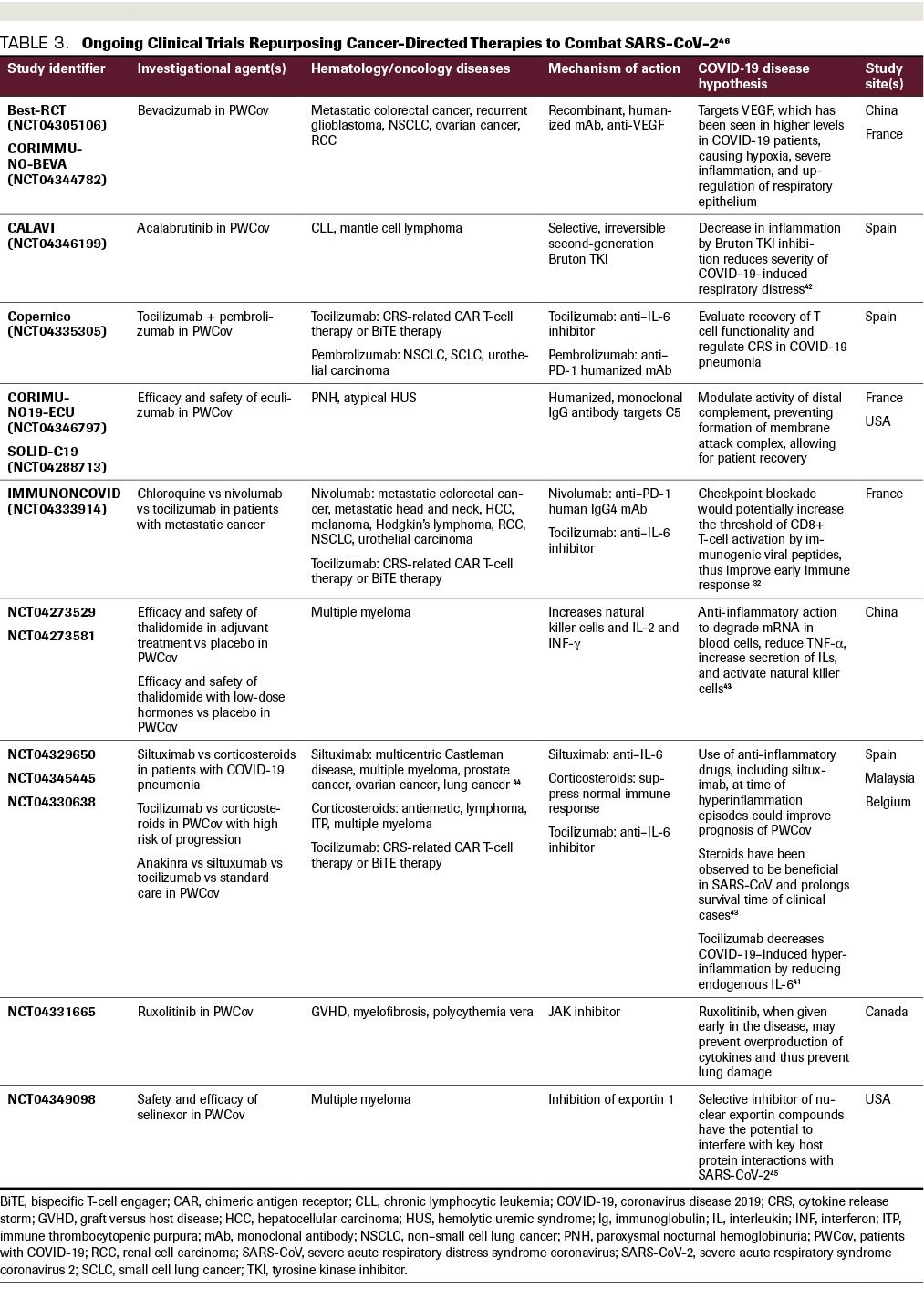
Many global clinical trials are underway to repurpose existing medications and evaluate the ways in which COVID-19 can be targeted to reduce viral entry or replication, or the excess inflammatory response and CRS (Table 3).31 The role of the PD-1/PD-L1 axis, which plays a fundamental role in the evasion of tumor cells from humoral immunity, has been well described in many solid malignancies such as lung cancer, renal cancer, bladder cancer, and melanoma. Viral infections such as HIV, hepatitis C, and hepatitis B also upregulate PD-1 and PD-L1 during acute and chronic infection, suggesting a potential role for PD-1/PD-L1 antagonists in the treatment of COVID-19 infection.32 However, concern has also been raised regarding the potential for these and other immunostimulatory agents to worsen disease by promoting CRS. The Bruton TKI ibrutinib, which modulates B-cell function, has shown potential to provide protection against COVID-19–induced lung injury in a small subset of patients with lymphoplasmacytic lymphoma.33 Other drugs approved for the treatment of hematologic and oncologic conditions that are being actively investigated for their potential benefits in COVID-19 illness include bevacizumab, an anti-VEGF used in multiple cancer therapies; eculizumab, an IgG antibody targeting C5 for paroxysmal nocturnal hemoglobinuria; and selinexor, an inhibitor of exportin 1 used in refractory multiple myeloma.
Logistical Modifications to Cancer Care During the COVID-19 Pandemic
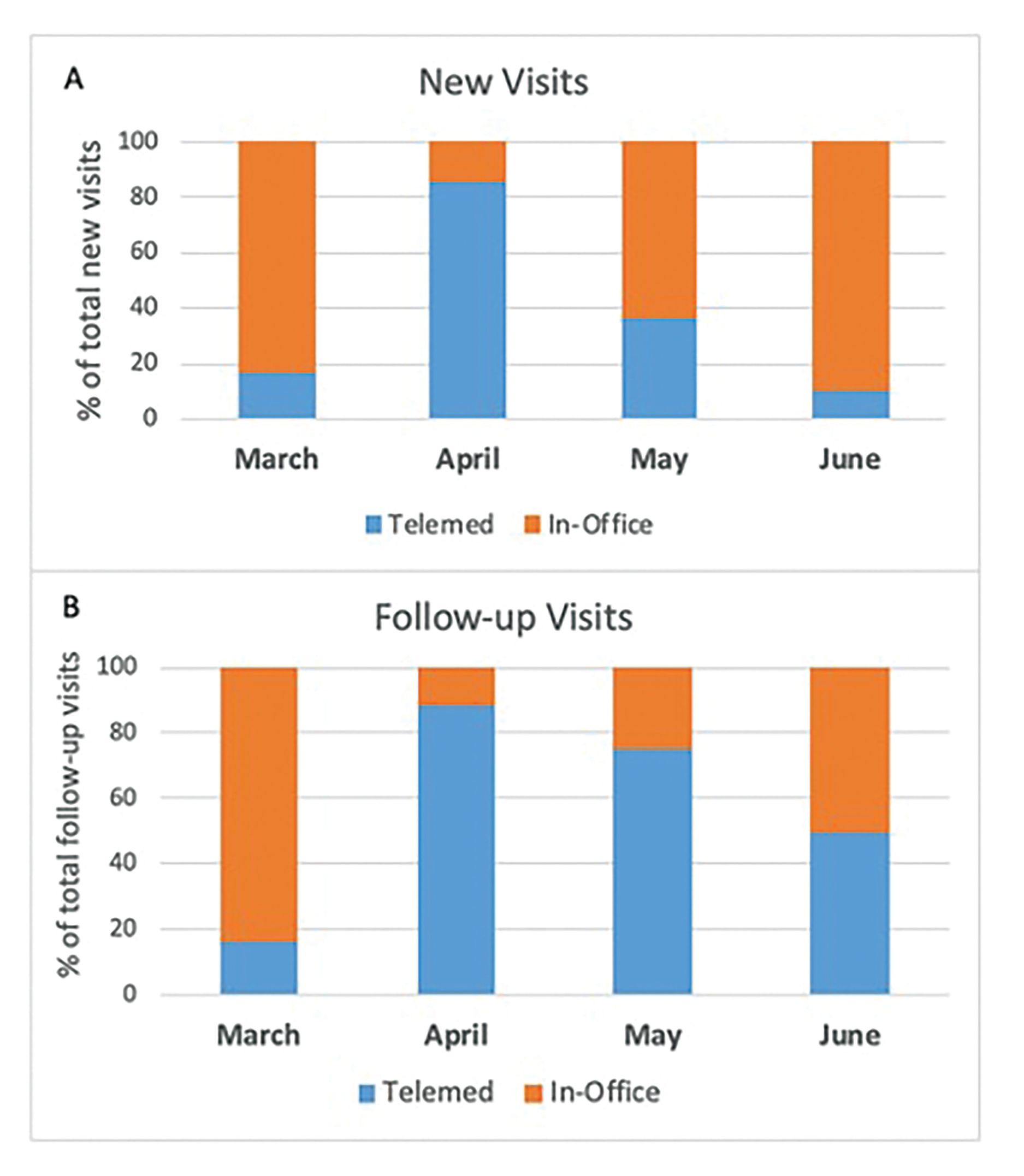
Outpatient visits. At the peak of the pandemic between March and April 2020, upward of 97% of inpatient admissions at our institution were patients with confirmed or suspected COVID-19. Several adaptations were required to protect both patients and staff, while assuring continuity of cancer care; many of these adaptations continue.11,34 Our clinic remained open, but more stringent personal protection provisions and infusion center policies were instituted, including screening all patients with temperature checks, symptom/risk questionnaires at facility entrances, and strict limitations on entry by nonpatient chaperones. Systemwide policies were instituted to convert most visits to billable virtual telemedicine visits. Patients were able to schedule video telehealth visits directly through their smartphones or tablets through our hospital’s secure app, and an increase in these visits was noted (Figure 2). Where possible, those patients who could not access the app were followed via telephone visits without a video component.
Figure 2. Trend Toward Increased Use of Telemedicine. This trend applied for both new patient and follow-up visits at our institution during the peak of the coronavirus disease 2019 epidemic in New York in April 2020.
For cancer patients who required in-person evaluation, such as those requiring a physical exam to survey cancer growth, temperatures were checked before entry. They were asked screening questions, including those regarding the presence of muscle aches; unusual fatigue; flulike symptoms or fever within the past 5 days; cough or shortness of breath; sick contact; and travel or contact with those who have traveled. Either an affirmation to any of these questions or an infrared temperature of 99.5 ºF or greater warranted a separate physician evaluation in a designated screening area. Patients with concerning signs or symptoms were directed to urgent care or the emergency department for SARS-CoV-2 nasal swab testing. For patients who were actively on cancer treatment who tested positive for SARS-CoV-2, policies were instituted describing when they could resume treatment. To restart treatment after testing positive, patients were required to be self-quarantined for 14 days and to be afebrile for at least 72 hours. If a staff member had been potentially exposed to COVID-19, a hotline was provided to discuss starting postexposure prophylaxis empirically with hydroxychloroquine when these studies were still ongoing.
Supportive medications. Several modifications to cancer care were implemented at our center to enhance safety during the pandemic, in line with guidelines for best practice.35 Most supportive care treatments, such as bone-modifying agents (eg, denosumab or zoledronic acid) or maintenance intravenous iron infusions for iron-deficient patients, were delayed so long as they were deemed nonessential. In addition, routine cancer screening tests, such as mammograms and colonoscopies, and bone density tests and restaging CT scans were also temporarily delayed. Genetic screenings for hereditary cancer syndromes like Lynch syndrome or BRCA mutations were done with telehealth visits and swabs sent to patients’ homes. The long-term consequences of these measures during the pandemic on cancer detection and prevention remain to be determined.
Cancer-specific modifications. The decision to treat an individual patient with myelosuppressive/immunosuppressive cancer therapies seemingly conflicts with strategies to improve infection control and decrease patient and staff exposure to SARS-CoV-2. Therefore, we have had to assess whether treatment plans be initiated on the usual schedules or delayed. For new patients with cancer at low risk of acute progression, such as asymptomatic low-grade lymphomas, treatment was generally delayed based on the diagnosis, cancer stage and presentation, goals of treatment, and an informed conversation with patients about the potential risks of cancer treatment during the pandemic. At our institution, new enrollments into clinical trials were temporarily halted to limit exposure to infections, and also as a consequence of allocation of ancillary research staff to other COVID-19–related responsibilities, such as screening patients at entrances to various clinical centers. Similarly, new stem cell transplants were delayed when possible due to the need for inpatient beds. European Society for Blood and Marrow Transplantation guidelines recommended freezing a secured stem cell product until it becomes appropriate for conditioning.11 Diagnostic procedures such as biopsies were permitted; however, most elective procedures, such as embolizations, ablations, staging endoscopies and laparoscopies, and indwelling catheters, as well as invasive pain management measures, such as epidural pain pumps, were temporarily delayed.
TABLE 3. Ongoing Clinical Trials Repurposing Cancer-Directed Therapies to Combat SARS-CoV-2
Elective surgeries posed a challenge due to limited staffing and operating room space. Such procedures were placed on hold unless absolutely necessary due to acute symptoms. For those essential procedures, patients were tested for SARS-CoV-2 three days prior to surgery and, if positive, the surgery was delayed for 14 days and then conducted in an operating room designated for additional COVID-19 precautions such as airborne protections. As surgeries were deferred due to resource allocation, lower-stage cancers that are typically managed with resection, such as breast and lung cancers, were temporarily treated with neoadjuvant treatment in certain instances until surgery could be instituted. For example, neoadjuvant systemic therapy was recommended for early-stage gastric and esophageal cancer patients in accordance with management guidelines published in the Annals of Surgical Oncology.36 Similarly, for patients with newly diagnosed early-stage colon cancer who were unable to go immediately for surgery, neoadjuvant chemotherapy was used following guidelines published by the National Comprehensive Cancer Network.37 For hormone-positive DCIS or breast cancer, we often treated patients with endocrine therapy upfront, as was also suggested by several societies.38 Our proposed approach for triple-negative and HER2+ invasive breast cancers was to treat with neoadjuvant chemotherapy for all lower-stage disease in an effort to delay surgery for up to 4 to 8 weeks in resource-deprived settings, according to guidelines published in the Annals of Surgical Oncology.36 Similarly, for some lower-risk prostate cancers, hormonal therapy was recommended until surgery could be safely pursued.
Management of patients on immunotherapies was taken on a case-by-case basis. Immune checkpoint inhibitor (ICI)-related pneumonitis occurs in 2.5% to 5% of patients receiving anti–PD-1/PD-L1 monotherapy and in 7% to 10% of those on anti–CTLA-4/anti–PD-1 combination therapy, and it is the most common fatal adverse event in patients receiving these therapies.39 Given the extent of lung damage that is caused by COVID-19, and the possibility of pneumonitis associated with ICIs, we recognized a major clinical challenge in the timing of resumption of ICI therapies following recovery from COVID-19 illness.40 At a minimum, all patients resuming ICIs were required to demonstrate, on CT, stabilization or improvement in COVID-19–related chest findings, as well as declining inflammatory markers associated with CRS. At our institution, we did not withhold immunotherapy from cancer patients who were deemed to be benefiting and were without evidence of active or recent prior SARS-CoV-2 infection.
Lifesaving surgeries—such as those for intestinal perforation secondary to cancer, bowel obstruction from cancer, or acute hemorrhage from cancer with transfusion dependence—have been uninterrupted. Similarly, radiation therapy for emergent situations such as spinal cord compression, symptomatic brain metastasis, bleeding, airway compromise, or uncontrollable pain were not delayed during the pandemic. Patients with active SARS-CoV-2 infection requiring radiation therapy under such circumstances were scheduled as “last case of the day” followed by terminal facility cleaning prior to next-day operations.
It was and remains vital that we help patients make treatment decisions that are in line with their goals of care for themselves, while setting realistic expectations regarding prognosis with mechanical ventilation or with prolonged delays in treatment. We also communicated to patients that a prolonged delay to seeking care due to a fear of hospitalization could paradoxically lead to prolonged hospitalizations and have detrimental effects on their cancer care.
Given the major impact that COVID-19 has had on our oncology patients, clinical trials are underway to assess the changes that have occurred within this particular population
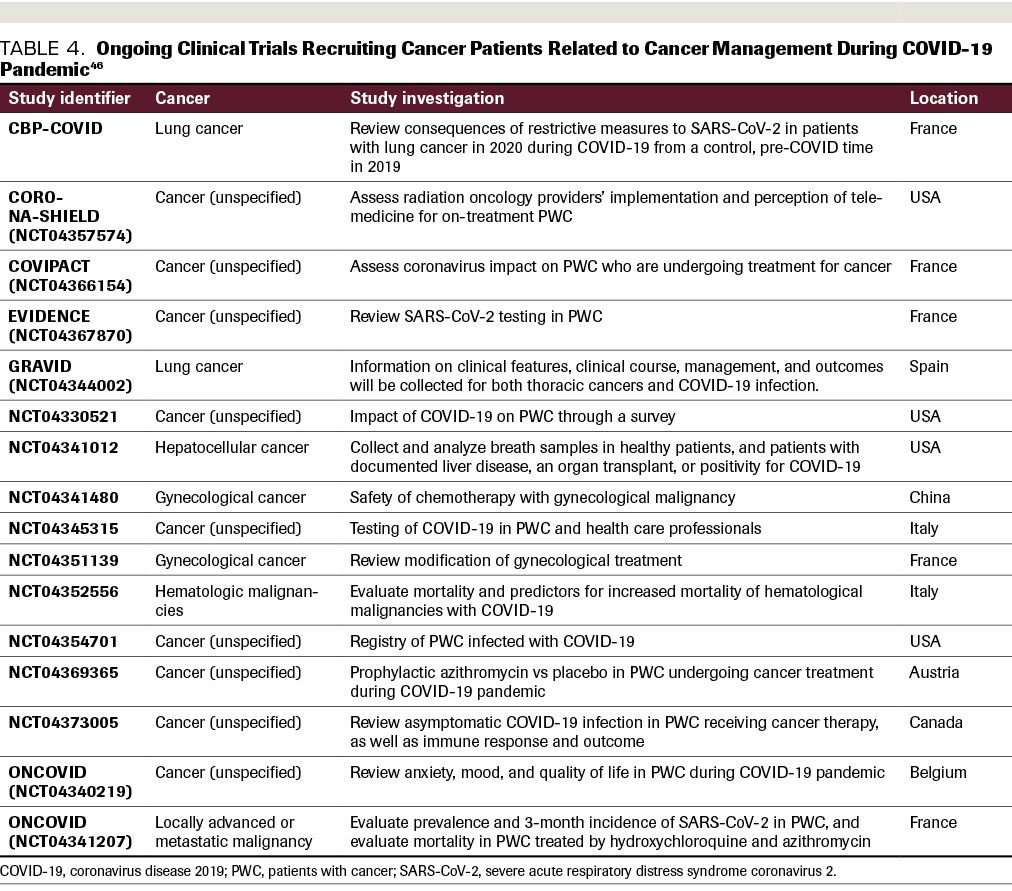
(Table 4). Sixteen clinical trials were either actively recruiting cancer patients or planning imminent recruitment to address specific concerns related to cancer management during the COVID-19 pandemic: 3 are designed to evaluate unique psychosocial issues of cancer patients during COVID-19; 3 are related to patient testing for COVID-19; 3
address specific changes to standard cancer treatment in recognition of COVID-19 concerns; 2 are designed to create a registry for cancer patients infected with COVID-19; 1 reviews the use of telemedicine visits for patients receiving radiation therapy; and the last looks at azithromycin prophylaxis for cancer patients on active therapy.
TABLE 4. Ongoing Clinical Trials Recruiting Cancer Patients Related to Cancer Management During COVID-19 Pandemic
Conclusions
Many challenges exist in caring for oncology patients during the COVID-19 pandemic, including balancing the benefits of oncology-directed treatment with the implicit increased risks of severe COVID-19 infection. Encouragingly, the rapid sequencing of the SARS-CoV-2 virus, development of a quantitative polymerase chain reaction test to detect infected individuals, evidence-based intensive care measures, and more than 500 clinical trials examining potential strategies to treat COVID-19 will all likely decrease harm to cancer patients and the general population during this pandemic. As more data come to light on the pathophysiology of the novel coronavirus, we will be able to better modify cancer care and treatment protocols to minimize risks to our patients. Until then, best practice in oncology care mandates strict infection control processes and informed conversations with our patients in which we discuss the risks and benefits of modifying standard approaches in order to decrease the risk of COVID-19 infection and its complications. We hope that our experience and perspective from relatively early in this pandemic will help to inform others for whom the worst may still lie ahead.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service pertaining to this article.
References
1.WHO Timeline - COVID-19. World Health Organization 27 April 2020. (Accessed 2020/06/20, at https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19.)
2.Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res 2020;7:11.
3.Li L, Zhang W, Hu Y, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA 2020.
4.WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. (Accessed 2020/06/28, at https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.)
5.Cumulative Cases by Days Since 50th Confirmed Case. Johns Hopkins University Medical Center, 2020. (Accessed 2020/09/09, at https://coronavirus.jhu.edu/data/cumulative-cases.)
6.Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol 2020;38:10-8.
7.Center for Disease Control and Prevention. Groups at Higher Risk for Severe Illness. May 14, 2020. (Accessed 2020/06/18, at https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html.)
8.Falandry C, Filteau C, Ravot C, Le Saux O. Challenges with the management of older patients with cancer during the COVID-19 pandemic. J Geriatr Oncol 2020.
9.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335-7.
10.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Annals of Oncology.
11.Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol 2020.
12.Curigliano G, Banerjee S, Cervantes A, et al. Managing cancer patients during the COVID-19 pandemic: An ESMO Interdisciplinary Expert Consensus. Ann Oncol 2020.
13.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844-7.
14.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020.
15.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020.
16.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020;9:727-32.
17.Marietta M, Vandelli P, Mighali P, et al. Randomised controlled trial comparing efficacy and safety of high versus low Low-Molecular Weight Heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials 2020;21:574.
18.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565-74.
19.Deming ME, Chen WH. COVID-19 and Lessons to be Learned from Prior Coronavirus Outbreaks. Ann Am Thorac Soc 2020.
20.Ceribelli A, Motta F, De Santis M, et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun 2020:102442.
21.Kong Q, Xiang Z, Wu Y, Gu Y, Guo J, Geng F. Analysis of the susceptibility of lung cancer patients to SARS-CoV-2 infection. Mol Cancer 2020;19:80.
22.Zhang Y, Li J, Zhan Y, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun 2004;72:4410-5.
23.Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol 2020.
24.Shen C, Wang Z, Zhao F, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020;323:1582-9.
25.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and Efficacy of INCB018424, a JAK1 and JAK2 Inhibitor, in Myelofibrosis. New England Journal of Medicine 2010;363:1117-27.
26.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet 2020;395:e30-e1.
27.Sisk JM, Frieman MB, Machamer CE. Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors. J Gen Virol 2018;99:619-30.
28.Shin JS, Jung E, Kim M, Baric RS, Go YY. Saracatinib Inhibits Middle East Respiratory Syndrome-Coronavirus Replication In Vitro. Viruses 2018;10.
29.Junmei W. Fast Identification of Possible Drug Treatment of Coronavirus Disease -19 (COVID-19) Through Computational Drug Repurposing Study2020.
30.Dyall J, Coleman CM, Hart BJ, et al. Repurposing of Clinically Developed Drugs for Treatment of Middle East Respiratory Syndrome Coronavirus Infection. Antimicrobial Agents and Chemotherapy 2014;58:4885-93.
31.Global Coronavirus COVID-19 Clinical Trial Tracker. (Accessed 2020/07/08 at https://www.covid-trials.org/.
32.Schönrich G, Raftery MJ. The PD-1/PD-L1 Axis and Virus Infections: A Delicate Balance. Frontiers in Cellular and Infection Microbiology 2019;9.
33.Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 2020;135:1912-5.
34.Ueda M, Martins R, Hendrie PC, et al. Managing Cancer Care During the COVID-19 Pandemic: Agility and Collaboration Toward a Common Goal. J Natl Compr Canc Netw 2020:1-4.
35.Wang Z, Wang J, He J. Active and Effective Measures for the Care of Patients With Cancer During the COVID-19 Spread in China. JAMA Oncology 2020.
36.Bartlett DL, Howe JR, Chang G, et al. Management of Cancer Surgery Cases During the COVID-19 Pandemic: Considerations. Annals of Surgical Oncology 2020.
37.COVID-19 Resources. National Comprehensive Cancer Network. (Accessed 2020/07/10 at https://www.nccn.org/covid-19/.)
38.Resource for Management Options of Breast Cancer During COVID-19. Society of Surgical Oncology 2020.
39.Russano M, Citarella F, Napolitano A, et al. COVID-19 pneumonia and immune-related pneumonitis: critical issues on differential diagnosis, potential interactions, and management. Expert Opin Biol Ther 2020:1-5.
40.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 2020;12:269-73.
41.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol 2020.
42.AstraZeneca. AstraZeneca initiates CALAVI clinical trial with Calquence against COVID-19. 2020.
43.Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica 2020;44:e40.
44.Chen R, Chen B. Siltuximab (CNTO 328): a promising option for human malignancies. Drug Des Devel Ther 2015;9:3455-8.
45.Ternyila D. A Global Randomized Trial Planned for Selinexor to Treat COVID-19. Targeted Oncology 2020.
46.NIH, ClinicalTrials.gov, U.S. National Library of Medicine (Accessed 2020/07/08, at https://clinicaltrials.gov/)

