Early-Stage BRCA2-Linked Breast Cancer Diagnosed in the First Trimester of Pregnancy Associated With a Hypercoagulable State
This feature examines the case of a patient with newly diagnosed breast cancer in the setting of a first-trimester pregnancy presenting to our multidisciplinary breast cancer clinic.
SECOND OPINION
Multidisciplinary Consultations on Challenging Cases
The University of Colorado Denver School of Medicine faculty holds weekly second opinion conferences focusing on cancer cases that represent most major cancer sites. Patients seen for second opinions are evaluated by an oncologic specialist. Their history, pathology, and radiographs are reviewed during the multidisciplinary conference, and then specific recommendations are made. These cases are usually challenging, and these conferences provide an outstanding educational opportunity for staff, fellows, and residents in training.
The second opinion conferences include actual cases from genitourinary, lung, melanoma, breast, neurosurgery, gastrointestinal, and medical oncology. On an occasional basis,
ONCOLOGY
will publish the more interesting case discussions and the resultant recommendations. We would appreciate your feedback; please contact us at
second.opinion@uchsc.edu
.
E. David Crawford, MD
Al Barqawi, MD
Guest Editors
University of Colorado Health Sciences Center
and Univeristy of Colorado Cancer Center
Denver, Colorado
This feature examines the case of a patient with newly diagnosed breast cancer in the setting of a first-trimester pregnancy presenting to our multidisciplinary breast cancer clinic.
Case Presentation
A 30-year-old female at 6 weeks’ gestation of a highly desired pregnancy presented to our multidisciplinary breast cancer clinic with a new diagnosis of invasive ductal carcinoma of the right breast. The patient had just become aware of her pregnant state when she noted a mass in her right breast on self-examination. She was evaluated with a diagnostic mammogram with proper shielding and an ultrasound, confirming a 1.4-cm solid mass corresponding to the palpable lesion (Figures 1 and 2). Ultrasound-guided core needle biopsy was obtained and revealed a grade 3/3 invasive ductal carcinoma, estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and human epidermal growth factor receptor 2 (HER2)-negative. On physical exam, she showed changes to the breast tissue consistent with early pregnancy, a palpable mass at the biopsy site, and no axillary adenopathy or contralateral findings. The remainder of her examination was likewise benign.
FIGURE 1
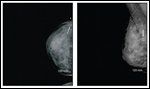
Diagnostic MammogramFIGURE 2
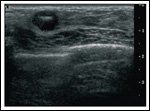
Ultrasound ConfirmationTABLE 1

Testing for Hypercoagulability
The patient’s past medical history was remarkable for a recent pregnancy loss at 12 weeks’ gestation 2 months prior. Her family history was pertinent for a strong family history of deep venous thrombosis in two first-degree relatives, and a breast cancer type 2 susceptibility protein (BRCA2)-associated breast cancer in a paternal aunt. The patient had not been tested for the familial BRCA2 gene mutation prior to her breast cancer diagnosis.
After her 12 weeks’ pregnancy loss, the patient had undergone workup for a hypercoagulable state, which is summarized in Table 1. Testing showed a low protein S activity level on two separate occasions and the presence of a double heterozygote state for mutations in the methylene tetrahydrofolate reductace (MTHFR) gene. Her homocysteine level was below the lower limit of normal. The patient denied a personal history of deep venous thrombosis or arterial thrombosis and was not receiving systemic anticoagulation.
Discussion
Diagnostic Testing in Pregnancy
Dr. Jennifer Diamond: Ultrasound is ideal in evaluating a breast mass during pregnancy as it poses no risk to the fetus and has a sensitivity for malignancy approaching 100%.[1-3] Mammography also poses little risk to the fetus and is considered to be a safe imaging technique in pregnancy if necessary. The dose of radiation to the fetus with a standard bilateral mammography using abdominal shielding has been estimated at 0.004 Gy of radiation, falling well below the threshold for malformation in the fetus of 0.05 Gy of radiation.[4] The earlier in pregnancy, the lower the exposure to the fetus.
If a hypoechoic mass concerning for malignancy is seen on ultrasound in the setting of pregnancy, is mammography necessary?
Dr. Laura Hardesty: Mammography is recommended for several reasons in this scenario. Although it is known that cancer is present at the site of the palpable mass, evaluation of the remainder of the ipsilateral breast and of the contralateral breast for the presence of additional sites of malignancy is necessary. Mammography allows for a “whole breast” view that cannot be obtained by sonography. Even if screening sonography of the entirety of both breasts is performed, only small portions of the breasts are imaged at one time. The radiologist must mentally “synthesize” a summary view of the breasts. As a result, mammography may provide a more complete understanding of the three-dimensional location of the cancer within the breast, particularly if there is more than one mass suspicious for cancer.
In addition, mammography is markedly more sensitive than sonography for detecting small calcifications that may indicate malignancy extending beyond the size of the sonographically imaged tumor, often ductal carcinoma in situ (DCIS). The difference in tumor size detectable by mammography vs sonography may also be significant, thus affecting treatment decisions regarding lumpectomy vs mastectomy.
In general, the mammographic density of the breasts increases with pregnancy-induced breast changes. This decreases the sensitivity for detecting breast masses, but does not decrease the ability to detect cancer-associated calcifications. The increase in mammographic glandular density becomes more marked as the pregnancy progresses, and is unlikely to be a limiting factor at 6 weeks’gestation
Dr. Diamond: Is there a role for breast magnetic resonance imaging (MRI) in the evaluation of the pregnant patient with newly diagnosed breast cancer?
Dr. Hardesty: The short answer is no. Unenhanced MRI of the breast could be performed during pregnancy; however, it is not useful for the evaluation of breast cancer. Intravenous gadolinium is contraindicated at any time during pregnancy, as it is known to cross the placenta and causes fetal abnormalities in animal models.[5,6]
Dr. Diamond: Radiographic staging exams are recommended in newly diagnosed stage IIB or higher cases or in the setting of concerning symptoms, physical exam findings, or laboratory abnormalities. What studies can safely be performed in pregnancy?
Dr. Hardesty: Sonography of the axilla is useful in screening for metastatic axillary lymphadenopathy, with subsequent ultrasound-guided core biopsy of suspicious axillary lymph nodes. Chest radiography can be performed with abdominal/pelvic lead apron shielding of the pregnancy. Sonography of the liver and/or MRI of the spine without gadolinium could also be performed at no risk to the fetus if clinically indicated.
Dr. Virginia Borges: In this patient who has a clinical T1, N0 tumor, I would recommend no alteration from the usual guidelines: checking hepatic function, a complete blood count with differential, and a chest x-ray with proper shielding for preoperative staging. A chest x-ray is recommended by National Comprehensive Cancer Network (NCCN) guidelines for staging of all newly diagnosed breast cancer, though published rates of actual detection of metastasis are between 0.93% and 1.2%.[7,8] If hepatic function is abnormal, then a liver ultrasound would also be recommended.
Surgical Management of Breast Cancer in Pregnancy
Dr. Diamond: How does a first-trimester pregnancy affect your surgical decision-making?
Dr. Christina Finlayson: Approximately 1% to 2% of pregnant women require a nonobstetric surgery during their pregnancy. Extensive experience has demonstrated that anesthesia at all stages of pregnancy is safe for both the mother and the fetus.[9] Therefore, surgical therapy decisions for pregnancy-associated breast cancer can be made based on the oncologic situation, without the need for modification based on anesthetic concerns.
Sentinel lymph node sampling during pregnancy, however, is a controversial topic. Isosulfan blue dye and methylene blue dye are vital dyes and pregnancy category C drugs, while radiocolloid poses a radiation risk to the fetus. It is accepted that the vital blue dyes should not be used during pregnancy for sentinel lymph node localization, but the use of radiocolloid during pregnancy has been reported in the literature with modeling in nonpregnant women estimating the radiation dose to the fetus to be below the threshold for malformation.[10,11] A very small series of patients given a radioactive tracer showed successful localization of the sentinel lymph node without adverse fetal outcome.[12] From the currently available literature, localization of the sentinel lymph nodes with radioactive tracer alone appears to be a safe procedure. For mothers who do not want to assume the theoretical risk of early radiation exposure to the fetus, an axillary node dissection continues to be an excellent and appropriate technique for staging the axilla.
Dr. Diamond: The family history of BRCA2-associated breast cancer and young age at diagnosis make a genetic cancer syndrome likely in this patient. Would the presence of a BRCA2 mutation change your surgical recommendations for this patient?
Dr. Finlayson: Patients with a known BRCA1 or BRCA2 mutation have similar outcomes with local therapy as patients without a known gene defect. However, they have a much higher risk of subsequent cancers than patients without a genetic predisposition.[13] The risk of a second cancer in the contralateral breast within the next 10 years can be as high as 40% to 50% in some series.[13-15] Therefore, many women select mastectomy to treat their known cancer, and contralateral prophylactic mastectomy for maximal risk reduction against a second breast cancer.
In an international cohort of BRCA1/2 gene mutation carriers with breast cancer, the rate of contralateral prophylactic mastectomy was 27% overall, and 49% in North American women. In this cohort, women choosing contralateral prophylactic mastectomy were younger at the time their cancer was diagnosed, and more likely to undergo ipsilateral mastectomy for the treatment of their known cancer.[16]
In this young woman with a family history of a BRCA2 gene mutation, the decision for breast conservation vs mastectomy to treat her known breast cancer will likely be influenced by the results of her genetic testing. In this situation, lumpectomy and nodal sampling can be performed followed by initiation of systemic therapy as appropriate while her genetic testing is being performed. Radiation therapy, however, should not be initiated until after the genetic testing results are available, and the patient has decided to forgo mastectomy due to either personal choice or negative gene mutation results.
Postlumpectomy whole-breast irradiation followed by mastectomy should be avoided, as it subjects patients to unnecessary treatment, is redundant, and can complicate potential reconstructive options. Prosthetic reconstruction in the setting of radiation therapy to the breast or chest wall is associated with an increase in complications including capsular contracture and infection.[17,18] Autologous reconstruction after radiation therapy can also lead to decreased aesthetic outcomes and increased contracture rates.[19]
Radiation Therapy in the Setting of Pregnancy-Associated Breast Cancer
Dr. Diamond: While this patient has a known BRCA2 mutation in her family and should undergo genetic testing, she may be a candidate for breast-conservation surgery upfront based on the size of the tumor. How does pregnancy change your recommendations for postlumpectomy radiation?
Dr. Rachel Rabinovitch: I agree with Dr. Finlayson and would not initiate radiotherapy for this patient until her genetic testing results are returned and she has made a final decision to forgo mastectomy, pregnancy aside.
With regard to the patient’s pregnancy, the radiation dose exposure to a fetus resulting from breast radiotherapy is a serious issue. Exposure to the fetus during breast radiotherapy results from internal scatter, leakage radiation from the tube head of the linear accelerator, and scatter from the collimator and other machine components. There is no dose below which the consequences of radiation exposure to the fetus are nonexistent. Radiation exposure to the fetus from 8 to 25 weeks gestation primarily exposes the child to risks of central nervous system damage, which can manifest clinically from mildly decreased intelligence to profound mental retardation, depending on dose and timing of exposure.[20] In utero radiation exposure at any time places the child at an increased lifetime risk of developing a malignancy.[20]
The anticipated dose to the fetus from 50 Gy of whole-breast radiotherapy is expected to be roughly 0.1% to 0.3% of the total dose. The threshold dose for mental retardation resulting from exposure during gestational weeks 16 to 25 is 0.25 Gy, a dose that can be reached during routine breast radiotherapy. Radiation exposure increases in later pregnancy as the fetus grows and lies closer to the treated breast. As such, the indication for and benefits of radiotherapy to the patient, alternative treatment options, and potential consequences of treatment to the fetus must all be considered.
While breast conservation is the preferred treatment approach for early-stage breast cancer in most women, here the best options would include either a planned mastectomy or delay of radiotherapy until after the fetus can be safely delivered. In most cases of early breast cancer treated with breast-conservation therapy, this delay in initiation of breast radiotherapy maintains treatment efficacy for the patient paired with safety to the fetus.
Dr. Diamond: Our patient expressed a strong desire to breast-feed her child. What would you advise her if she strongly desires breast conservation, and radiotherapy could be delayed until after delivery?
Dr. Rabinovitch: If our patient is BRCA2 gene normal, or declines bilateral mastectomies after appropriate counseling in the setting of a gene mutation, then breast radiotherapy after delivery is indicated. While there are no formal guidelines in this setting, I have found it most straightforward to encourage the patient to initiate breast-feeding only on the contralateral (normal) side. This will result in suppression of lactation in the index breast, and allow for a reasonably stable target organ size for radiation treatment planning within 2 to 3 weeks of delivery. It also avoids complicating breast irradiation with the potential concerns related to mastitis, breast engorgement, irradiated breast milk, and additive causes of nipple and breast tenderness. I would advise her of the potential for significant breast asymmetry for the duration of breast feeding, as the lactating breast can become much larger than the other.
Furthermore, I would advise her to inform the infant’s pediatrician of the plan for unilateral lactation, as infants may require some form of supplementation to meet normal growth milestones in this setting.
Unilateral lactation has been studied in women with a history of breast-conservation surgery and radiation therapy prior to pregnancy. Normal lactation can occur 20% to 25% of the time in a previously irradiated breast, although more commonly the irradiated breast has significantly less swelling during pregnancy and less milk production.[21,22] The patient should be informed of this risk for future pregnancies and reminded that her breast milk is not radioactive and will not harm her child.
Systemic Chemotherapy in the Setting of Pregnancy
Dr. Diamond: How does a first-trimester pregnancy affect your recommendations for adjuvant chemotherapy in the treatment of this patient’s early-stage breast cancer? Would you discuss terminating the pregnancy with this patient?
Dr. Peter Kabos: This is a highly desired pregnancy, and the presentation with clinical stage I disease makes pursuing treatment of the cancer while maintaining the pregnancy a reasonable option. Details of how to approach such treatment have been expertly reviewed and are available as published guidelines.[23-28]
The recommendation for systemic chemotherapy is based on the same estimation of recurrence risk and potential benefit as in a nonpregnant woman. However, chemotherapy should be withheld until after the first trimester due to the risk of significant fetal malformation. Cyclophosphamide and doxorubicin have been successfully used in the second and third trimesters of pregnancy without evidence of fetal harm. Individual series have noted some cases of intrauterine growth retardation and preterm birth, while administration within 1 month of delivery can result in low maternal and fetal blood counts and therefore should be avoided. Co-treated offspring with follow-up data demonstrate no subsequent complications from in utero chemotherapy exposure.[25,28-29]
In this case, based on high tumor grade, tumor size greater than 1 cm, triple-negative status, young age, and clinically lymph node–negative findings, I would recommend adjuvant chemotherapy with AC (doxorubicin [Adriamycin] and cyclophosphamide) for four cycles to begin after week 12. Ondansetron and low-dose dexamethasone can safely be used for prophylaxis and treatment of nausea. In our experience, pregnant women tend to have less chemotherapy-induced nausea, and lower requirements for antiemetics. Granulocyte colony-stimulating factor (Neupogen) has been used during pregnancy, and limited evidence suggests it is safe.[25,26,30] However, dose-dense regimens requiring growth factor support should not be used during pregnancy.
Antiendocrine therapy should not be used during pregnancy. Tamoxifen, the preferred antiendocrine therapy in premenopausal women, is a pregnancy category D drug that has been associated with teratogenicity both in animal models and in humans with congenital craniofacial defects and ambiguous genitalia reported.[31] Trastuzumab (Herceptin), a monoclonal antibody to HER2, should also not be used during pregnancy as it has been associated with oligo- or anhydramnios.[32] Bisphosphonates should not routinely be used during pregnancy as they can lead to neonatal hypocalcemia and have an impact on bone development.[33]
Dr. Anthony Elias: If the patient is found to have lymph node involvement at the time of surgery, I would add treatment with a taxane to her adjuvant therapy. A number of case reports have documented the use of taxanes during pregnancy with no significant untoward effects seen on the fetus. In a nice review of the literature, Mir et al reported nine cases of the use of paclitaxel after the second trimester and six reports of the use of docetaxel.[34] However, if this patient does have node-positive disease, I would prefer deferring taxane therapy to after delivery, until more safety data are available.
Dr. Diamond: Can you comment on the prognosis of pregnancy-associated breast cancer?
Dr. Borges: Pregnancy-associated breast cancer (PABC) is a term used to variably define breast cancer diagnosed during or up to a number of years after delivery of a child. Epidemiologic data support the concept that PABC encompasses two distinct subsets with differing prognoses: those diagnosed and treated while pregnant and those diagnosed and/or treated after delivery of a child.[35] For women who are diagnosed and started on treatment during pregnancy, there is no appreciable alteration of prognosis that is specific to the presence of the pregnancy when cases are otherwise matched on biologic characteristics known to drive prognosis. Diagnosis during pregnancy, however, is associated with a delay in diagnosis, larger tumor size, higher grade, and enrichment for hormone receptor–poor and HER2-overexpressed biology. Appropriate management of such cases appears to permit equivalent outcomes without requirement for termination of the pregnancy.[36]
Conversely, cases of breast cancer diagnosed within months to several years postpartum demonstrate a higher risk of metastasis for reasons that are currently being investigated.[37] The effect of worsened prognosis in the postpartum period shows a time-dependent curve, with closer proximity to parturition being associated with a higher risk, which gradually diminishes with a persistent tail effect out to even 15 years in one series.[35,38] How best to mitigate against this increased risk of metastasis with available therapies in newly diagnosed young mothers has yet to be elucidated.
In summary, breast cancer diagnosed during pregnancy may be a poorer-prognosis disease due to the biologic features and staging of the tumor at diagnosis, compared with other young women’s breast cancers. The maintenance of the pregnancy does not further worsen maternal outcome if the tumor can be otherwise appropriately managed. Distinction in the risk of metastasis between the two subsets of PABC are important when offering counseling to a newly diagnosed pregnant patient.
Prenatal Care for the Patient With Pregnancy-Associated Breast Cancer
Dr. Diamond: How do you change your recommended prenatal care in a patient receiving chemotherapy?
Dr. Linda Barbour: If the prescribed chemotherapy is associated with any risk of growth restriction, fetal loss, or preeclampsia, fetal surveillance in the form of serial fetal ultrasounds for growth and third-trimester nonstress tests should be offered. Fetal surveillance in the form of daily fetal activity records should also be encouraged beginning at 26 to 28 weeks. Coordination with a perinatologist is ideal to ensure that optimal maternal management includes appropriate laboratory testing or imaging studies, thromboembolism prophylaxis, and monitoring, and that the timing of delivery be optimized if maternal or fetal concerns warrant an earlier delivery. Chemotherapy should not be administered after 35 weeks’ gestation or within 2 to 3 weeks of the expected time of delivery, to prevent myelosuppression at the time of delivery.
Anticoagulation During This Pregnancy
Dr. Diamond: Can you comment on the diagnosis of protein S deficiency in this patient based on her prior hypercoagulable workup?
Dr. Christiane Thienelt: Even for a hematologist, protein S deficiency remains difficult to diagnose due to phenotypic variability and available testing methods. Plasma levels are influenced by a variety of factors including age, sex, oral contraceptive use, and pregnancy.[39] Protein S exists in two different forms: the functionally active free protein S, and the functionally inactive protein S complexed with C4b-binding protein. The level of free protein S antigen is the most reliable way of diagnosing the deficiency.[40] During pregnancy, women show a progressive decrease in free protein S antigen and total protein S, possibly related to increasing C4b-binding protein and/or a decrease in total protein S levels.[41] The most progressive decline appears to be in the second trimester, but reduced levels are seen as early as 6 weeks’ gestation. In addition, at 8 weeks postpartum, 15% of women do not have normal protein S levels.[42]
In this patient, the diagnosis of protein S deficiency is difficult to make with certainty based on protein S activity levels at the time of pregnancy loss and 2 months later. It would have been helpful to confirm the low protein S activity with a free protein S antigen level, and to have testing further out from her last pregnancy. It would also be very useful to know whether her relatives with thromboembolism had low protein S when at steady state (eg, no clot and no anticoagulation).
Dr. Diamond: Would you recommend prophylactic anticoagulation during her pregnancy?
Dr. Thienelt: Despite the uncertainty of her diagnosis of protein S deficiency, one must weigh the consequences of recurrent miscarriage and thrombotic complications against the risks of anticoagulation. This patient has numerous risk factors for venous thromboembolic complications, including a possible inherited hypercoagulable state based on laboratory evaluation, her family history, pregnancy, and a new diagnosis of malignancy.
In women with documented thrombophilia but no prior history of thrombosis, the American College of Chest Physicians guidelines for prevention and treatment of thromboembolic complications (8th edition) recommend antepartum surveillance or use of prophylactic dose low-molecular-weight heparin (LMWH)/unfractionated heparin (UFH) plus postpartum anticoagulants.[43] In light of this patient’s numerous risk factors for thrombosis and her history of miscarriage possibly caused or confounded by an underlying hypercoagulable state, I would recommend prophylaxis with LMWH throughout her pregnancy and postpartum.
Dr. Diamond: As a specialist in the medical complications of pregnancy, would you recommend prophylactic anticoagulation for this patient during her pregnancy based on the results of her prior hypercoagulable workup? What factors play into your decision?
Dr. Barbour: Women with an underlying thrombophilia such as protein S deficiency, but no known history of personal thrombosis, are at increased risk of thrombosis, but it is not clear whether this risk is high enough to warrant antepartum prophylaxis. Most experts would suggest that the risk/benefit ratio favors at least postpartum prophylaxis.[44,45] This is because thromboembolic events appear to occur more commonly in women with protein S deficiency, and the at-risk period is limited to 6 weeks postpartum.
It is also not clear whether women with a history of a 12-week pregnancy loss who are found to be protein S deficient warrant antepartum prophylaxis to decrease the risk for further pregnancy losses. However, one small randomized, controlled trial found that there may be a benefit to using daily LMWH in women with pregnancy loss after 10 weeks who were heterozygous for the factor V Leiden mutation, prothrombin gene mutation, or protein S deficiency.[46] Given that protein S deficiency has variable penetrance among families, it is recommended that the decision for antepartum prophylaxis be individually considered and that a family history of thrombosis, as well as other risk factors that might increase hypercoagulability, be considered.
In this particular patient, it appears that the protein S deficiency is real, because it was screened for outside of pregnancy and there is a documented family history of thrombosis. As Dr. Thienelt points out, however, the diagnosis is difficult to make. The double heterozygosity for MTHFR mutation has no clinical relevance since the patient’s homocysteine levels were low, likely due to folic acid supplementation in her prenatal vitamin, as only high levels are associated with pregnancy loss. Given that this patient has a strong family history of thrombosis in two first-degree relatives (suggesting strong penetrance of this trait), that she may be hypercoagulable to some degree due to her underlying malignancy, and that she has a history of a 12-week pregnancy loss, the benefits would seem to outweigh the risks for antepartum prophylaxis in her case.
Either daily or twice-daily prophylactic doses of LMWH could be offered (40 mg or enoxaparin or 5,000 IU of dalteparin), but there are no randomized controlled trials directly comparing the efficacy in thromboembolism prophylaxis using daily to twice-daily dosing. Since the patient has never had a personal history of thrombosis, and once-daily dosing was used to decrease the risk of recurrent fetal loss, I would favor daily LMWH prophylaxis to minimize risk.
Dr. Diamond: How is anticoagulation managed around delivery?
Dr. Barbour: If the patient is on daily LMWH, the injection can simply be held as soon as she experiences any contractions.
However, she may not be a candidate for epidural anesthesia if she has received an injection of LMWH within 12 to 24 hours of the time of planned epidural placement. Some obstetricians might recommend holding a dose for 24 hours and then scheduling an elective induction. However, this may increase the risk of a Cesarean delivery if her cervix is not favorable, in light of the fact that she has not previously delivered a full-term fetus. Cesarean delivery further increases the risk of thrombosis-especially pulmonary embolism-and should be avoided.
Alternatively she could be switched to low doses of unfractionated heparin near term to take advantage of its short half-life at doses that do not prolong the activated partial thromboplastin time (aPTT). She would be instructed to hold the dose for any contractions, and as long as the aPTT is not prolonged at the time of epidural placement, she would be a candidate for this approach. Within 12 to 24 hours after delivery, daily LMWH can be restarted. The patient could elect to stay on daily injections of LMWH for 6 weeks postpartum or be transitioned to warfarin. If she prefers to be switched to warfarin, which is also compatible with breast-feeding, she will require overlap with LMWH for at least 5 days and must also achieve an international normalized ratio (INR) of 2 to 3 on two occasions before the LMWH is stopped.
Treatment of BRCA1/2 Gene Mutation Carriers
Dr. Diamond: This patient’s triple-negative breast cancer is more consistent with a BRCA1 gene mutation, whereas BRCA2 gene mutation–associated tumors are more commonly ER/PR-positive. In a recent review of BRCA gene mutation–associated breast cancers, 57% of BRCA1-associated tumors were triple-negative compared to 23% of BRCA2-associated tumors.[47]
What are the current recommendations surrounding prophylactic mastectomy and oophorectomy in BRCA2 gene mutation carriers?
Dr. Catherine Klein: In both prospective and retrospective studies, prophylactic total mastectomy appears to reduce the risk of subsequent breast cancer by about 90%.[48] Ongoing trials will better address the question of survival benefit from this procedure, but preliminary studies suggest that cancer-related survival may also improve to as much as 80%. Case reports document new cancers in women who have undergone subcutaneous mastectomy, which leaves significant glandular tissue behind; hence, we recommend total mastectomy. The psychosocial complications of mastectomy, along with good options for cancer screening and for prevention with tamoxifen, have combined to make uptake of prophylactic mastectomy relatively low, compared to prophylactic oophorectomy.
For women who are over age 35 or have completed childbearing, bilateral salpingo-oophorectomy has been the standard recommendation. The risk reduction in ovarian cancer appears to be about 95%, and there is an additional breast cancer risk reduction of about 50%.[49] Unfortunately there is a small residual risk of primary peritoneal cancer.
Dr. Diamond: If this patient chooses to delay prophylactic surgery, how should she be screened for a second breast or ovarian cancer?
Based on expert opinion and not randomized studies, we recommend monthly breast self-examination beginning at about age 18, a clinical breast examination twice yearly beginning at age 25, and annual mammography with breast MRI at age 25 or about 10 years younger than the youngest case in the family.[50] The value in terms of early detection and prevention of breast cancer mortality rendered by this strategy is not known. CA-125 serum assay and transvaginal ultrasound are offered twice yearly beginning around age 30 for ovarian cancer early detection. Likewise, this strategy has not been shown to reduce ovarian cancer mortality.
Summary
This patient was found to have a BRCA2 gene mutation. She underwent lumpectomy and axillary lymph node dissection without any evidence of lymph node metastasis. Systemic chemotherapy with doxorubicin and cyclophosphamide for four cycles was administered beginning in the second trimester. She was treated with prophylactic LMWH until delivery and then for 6 weeks postpartum. She delivered a healthy baby boy and, after a period of breast-feeding, underwent bilateral mastectomy with immediate reconstruction. She remains well and is expecting her second child. Prophylactic oophorectomy is planned after completion of this pregnancy.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Liberman L, Giess CS, Dershaw DD, et al: Imaging of pregnancy-associated breast cancer. Radiology 191:245-248, 1994.
2. Ahn BY, Kim HH, Moon WK, et al: Pregnancy- and lactation-associated breast cancer: Mammographic and sonographic findings. J Ultrasound Med 22:491-497, 2003.
3. Yang W, Dryden M, Gwyn K, et al: Imaging of breast cancer diagnosed and treated during pregnancy. Radiology 239:52-60, 2006.
4. Greskovich JF Jr, Macklis RM: Radiation therapy in pregnancy: Risk calculation and risk minimization. Semin Oncol 27:633-645, 2000.
5. Pelsang RE: Diagnostic imaging modalities during pregnancy. Obstet Gynecol Clin North Am 25:287-300, 1998.
6. Nicklas AH, Baker ME: Imaging strategies in the pregnant cancer patient. Semin Oncol 27:623-632, 2000.
7. Puglisi F, Follador A, Minisini AM, et al: Baseline staging tests after a new diagnosis of breast cancer: Further evidence of their limited indications. Ann Oncol 16:263-266, 2005.
8. Ravaioli A, Pasini G, Polselli A, et al: Staging of breast cancer: New recommended standard procedure. Breast Can Res Treat 72:53-60, 2002.
9. Kuczkowski KM: The safety of anaesthetics in pregnant women. Expert Opin Drug Saf 5:251-264, 2006.
10. Spanheimer PM, Graham MM, Sugg SL, et al: Measurement of uterine radiation exposure from lymphoscintigraphy indicates safety of sentinel lymph node biopsy during pregnancy. Ann Surg Oncol 16:1143-1147, 2009.
11. Pandit-Taskar N, Dauer LT, Montgomery L, et al: Organ and fetal absorbed dose estimates from 99Tc-sulfur colloid lymphoscintigraphy and sentinel lymph node localization in breast cancer patients. J Nucl Med 47:1202-1208, 2006.
12. Mondi MM, Cuenca RE, Ollilia DW, et al: Sentinel lymph node biopsy during pregnancy: Initial clinical experience. Ann Surg Oncol 14:218-221, 2007.
13. Liebens FP, Carly B, Pastijn A, et al: Management of BRCA1/2 associated breast cancer: A systemic qualitative review of the state of knowledge in 2006. Eur J Cancer 43:238-257, 2007.
14. Haffty BG, Harrold E, Khan AJ, et al: Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet 359:1471-1477, 2002.
15. Metcalfe K, Lynch HT, Ghadirian P, et al: Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 22:2328-2335, 2004.
16. Metcalfe KA, Lubinski J, Ghadirian P, et al: Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: The Hereditary Breast Cancer Clinical Study Group. J Clin Oncol 26:1093-1097, 2008.
17. Percec I, Bucky LP: Successful prosthetic breast reconstruction after radiation therapy. Ann Plast Surg 60:527-531, 2008.
18. Behranwala KA, Dua RS, Ross GM, et al: The influence of radiotherapy on capsule formation and aesthetic outcome after immediate breast reconstruction using biodimensional anatomical expander implants. J Plast Reconstr Aesthet Surg 59:1043-1051, 2006.
19. Spear SL, Ducic I, Low M, et al: The effect of radiation on pedicled TRAM flap breast reconstruction: Outcomes and implications. Plast Reconstr Surg 115:84-95, 2005.
20. Kal HB, Struikmans H: Radiotherapy during pregnancy: Fact and fiction. Lancet Oncol 6:328-333, 2005.
21. Moran MS, Colasanto JM, Haffty BG, et al: Effects of breast-conserving therapy on lactation after pregnancy. Cancer J 11:399-403, 2005.
22. Tralins AH: Lactation after conservative breast surgery combined with radiation therapy. Am J Clin Oncol 18:40-43, 1995.
23. Carlson RW, Allred DC, Anderson BO, et al: Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 7:122-192, 2009.
24. Keleher AJ, Theriault RL, Gwyn KM, et al: Multidisciplinary management of breast cancer concurrent with pregnancy. J Am Coll Surg 194:54-64, 2002.
25. Molckovsky A, Madarnas Y: Breast cancer in pregnancy: A literature review. Breast Cancer Res Treat 108:333-338, 2008.
26. Lenhard MS, Bauerfeind I, Untch M: Breast Cancer and pregnancy: Challenges of chemotherapy. Crit Rev Oncol Hematol 67:196-203, 2008.
27. Navrozoglou I, Vrekoussis T, Kontostolis E, et al: Breast cancer during pregnancy: A mini-review. Eur J Surg Oncol 34:837-843, 2008.
28. Pereg D, Koren G, Lishner M: Cancer in pregnancy: Gaps, challenges and solutions. Cancer Treat Rev 34:302-312, 2008.
29. Hahn KM, Johnson PH, Gordon N, et al: Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer 107:1219-1226, 2006.
30. Calhoun DA, Christensen RD: A randomized pilot trial of administration of granulocyte colony-stimulating factor to women before preterm delivery. Am J Obstet Gynecol 179:766-771, 1998.
31. Berger JC, Clericuzio CL: Pierre Robin sequence associated with first trimester fetal tamoxifen exposure. Am J Med Genet A 146A:2141-2144, 2008.
32. Bader AA, Schlembach D, Tamussino KF, et al: Anhydramnios associated with administration of trastuzumab and paclitaxel for metastatic breast cancer during pregnancy. Lancet Oncology 18:79-81, 2007.
33. Levy S, Fayez I, Taquchi N, et al: Pregnancy outcome following in utero exposure to bisphosphonates. Bone 44:428-430, 2009.
34. Mir O, Berveiller P, Ropert S, et al: Emerging therapeutic options for breast cancer chemotherapy during pregnancy. Ann Oncol 19:607-613, 2008.
35. Lyons TR, Schedin PJ, Borges VF: Pregnancy and breast cancer: When they collide. J Mammary Gland Biol Neoplasia 14:87-98, 2009.
36. Beadle BM, Woodward WA, Middleton LP, et al: The impact of pregnancy on breast cancer outcomes in women 37. Schedin P: Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer 6:281-291, 2006.
38. Barnett GC, Shah M, Redman K, et al: Risk factors for the incidence of breast cancer: Do they affect survival from the disease? J Clin Oncol 26:3310-3316, 2008.
39. Henkins CM, Born VJ, Van der Schaaf W et al: Plasma levels of protein S, protein C, and factor X: Effects of sex, hormonal state and age. Thromb Haemost 74:1271-1275, 1995.
40. Makris M, Leach M, Beauchamp NJ, et al: Genetic analysis, phenotypic diagnosis, and risk of venous thrombosis in families with inherited deficiencies of protein S. Blood 95:1935-1941, 2000.
41. Clark P, Brennand J, Conkie JA, et al: Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 79:1166-1170, 1998.
42. Bremme K, Ostlund E, Almqvist I, et al: Enhanced thrombin generation and fibrinolytic activity in normal pregnancy and the puerperium. Obstet Gynecol 80:132-137, 1992.
43. Kearon C, Kahn SR, Agnelli G, et al: Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 133(6 suppl):454S-545S, 2008.
44. Bates SM, Greer IA, Pabinger I, et al: Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 133:844S-886S, 2008.
45. Barbour LA: Clinical Guidelines for Obstetricians and Gynecologists. Thromboembolism in Pregnancy, ACOG Practice Bulletin, no. 19, August 2000.
46. Gris JC, Mercier E, Quere I, et al: Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 103:3695-3699, 2004.
47. Atchley DP, Albarracin CT, Lopez A, et al: Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol 26:4282-4288, 2008.
48. Rebbeck TR, Friebel T, Lynch HT, et al: Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The PROSE study group. J Clin Oncol 22:1055-1062, 2004.
49. Rebbeck TR, Kauff ND, Domchek SM: Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophyrectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst 101:80-87, 2009.
50. Schwartz GF, Hughes KS, Lynch HT, et al: Proceedings of the International Consensus Conference on Breast Cancer Risk, Genetics, and Risk Management, April 2007. Breast J 15:4-16, 2009.