Fertility Preservation and Breast Cancer: A Complex Problem
A considerable number of women with breast cancer are diagnosed during their reproductive years. In the short period of time in which newly diagnosed women will need to make decisions about surgical options and adjuvant therapy, younger women with breast cancer also face the potential impairment or complete loss of their fertility.
A considerable number of women with breast cancer are diagnosed during their reproductive years. In the short period of time in which newly diagnosed women will need to make decisions about surgical options and adjuvant therapy, younger women with breast cancer also face the potential impairment or complete loss of their fertility. The preservation of fertility should therefore be an integral consideration in the choice of breast cancer therapy. Preserving ovarian function is particularly challenging for women with estrogen receptor– positive tumors, as the suppression of ovarian function has been shown to be beneficial therapy with regard to breast cancer outcomes. The current emerging recommendations of hormonal therapy extending to a 10-year period will likely further impact the timing of any subsequent pregnancy or require an interruption in hormonal therapy. To optimally counsel patients on how to best weigh the risks and benefits of each intervention, both the care provider and the patient must understand the options and the likelihood of their success. Here we summarize the current challenges and options for women diagnosed with breast cancer who wish to preserve their fertility.
Introduction
With improved early detection and treatment, the majority of women diagnosed with early-stage breast cancer will survive their disease. The majority of younger women will be offered adjuvant chemotherapy, and those whose tumors express estrogen receptors (ER-positive) should receive hormonal therapy for at least 5 years. Recent data suggest a benefit of extending hormonal therapy beyond 5 years, and often chemical ovarian suppression is added in young patients. As mortality has become less of an immediate threat, minimizing short-term and permanent long-term side effects should be a central goal when choosing adjuvant regimens. In addition to the risks of leukemia and heart failure, chemotherapy-induced premature ovarian failure must be considered. The insult to the ovaries by cytotoxic agents has been linked to the patient’s age at diagnosis, the type and dose of regimen used, likely genetic determinants of the host, and lifestyle factors.
There is still uncertainty about which are the most predictive biomarkers of poor ovarian reserve and impending infertility, and how to interpret their changes during adjuvant therapy for breast cancer. Several genetic markers predicting a higher likelihood of ovarian damage from treatment with specific chemotherapeutic agents are emerging and under clinical evaluation, but large randomized studies will be needed for validation.
The two most challenging groups of patients with whom to discuss fertility preservation are probably the patients whose tumors are ER-positive, as the induction of amenorrhea has been associated with better breast cancer–related outcomes, and patients with a genetic predisposition to ovarian cancer, such as women with mutations in BRCA1/BRCA2 or with Lynch syndrome (hereditary nonpolyposis colorectal cancer [HNPCC]) genes. In women with BRCA mutations, removal of the ovaries has been strongly associated with better breast cancer outcomes and prevention of ovarian cancer.
Little is still known about the emotional toll on patients who must navigate this very complex problem with conflicting priorities in a very short time. Access to novel technologies in fertility preservation prior to the start of chemotherapy is often not facilitated or thought feasible, because of perceived and actual risks to the patient, as well as costs and time constraints.
Care providers and patients should be informed about the risks, benefits, and likelihood of success in preserving ovarian function so they can make the best informed decision at a time that is stressful for the patient.
Fertility and natural risk factors for premature ovarian failure
Each year in the United States, more than 200,000 women are diagnosed with breast cancer,[1] of whom over 50,000 are under 50 years of age, with over 11,000 under age 40. Hence, at diagnosis an increasing number of young patients with breast cancer have not ever conceived or completed family planning.
One of the most important natural factors when considering fertility preservation is the age at breast cancer diagnosis and at the anticipated completion of adjuvant therapy. Changes in lifestyles over the last 3 decades have led to a delayed onset of childbearing, and there has been a steady increase in the number of children born to women over the age of 35. Nonetheless, the number of children born to women over age 35 is considerably lower than that in younger women. Whereas 97 children are born to 1,000 US women aged 30 to 34, this rate drops to 47 births at ages 35 to 39, to 10 at ages 40 to 44, and to 0.7 in women over 45 years of age, despite many recent advances in assisted reproductive technology (ART). ART has become an integral factor in childbearing. In 2011, an estimated 15 in 1,000 children in the United States were reported to be born through use of ART.[2]
Fertility is strongly linked to the onset of menopause. In the United States, the median age of menopause, the permanent cessation of menstruation and completion of natural fertility, is estimated to be 51 years; however, natural fertility rates decrease much earlier. A steady decrease in ovarian reserve and fertility is expected to occur after age 30, with a sharp drop after age 37.[3]
Premature menopause, defined as menopause occurring under the age of 40, is seen in about 1% of women. The likelihood of premature or early menopause (before age 45) is impacted by family history, smoking, ethnicity, and socioeconomic status.[4,5] These underlying risk factors should be considered in each patient when assessing the risk of chemotherapy-induced amenorrhea and the potential for successful fertility preservation.
Risk of chemotherapy-induced amenorrhea
The rate of chemotherapy-induced amenorrhea, the commonly used surrogate for infertility after chemotherapy and hormonal therapy, has been described in several excellent reviews.[6-9] Amenorrhea rates are frequently reported as secondary endpoints in large randomized studies evaluating novel therapeutic interventions for premenopausal patients with breast cancer. The induction of amenorrhea is dependent on the agent, dose, and duration of therapy; reported rates of post-treatment amenorrhea range from 10% with newer regimens and shorter durations of therapy to close to 100% in earlier studies. In addition to the aforementioned factors, the amenorrhea incidence rate is strongly associated with advancing age. Frequently, the reported data are further confounded by short follow-up of the respective study and the considerable reversibility of the chemotherapy-induced amenorrhea.
Several reports have suggested that addition of paclitaxel and trastuzumab (Herceptin) do not add to the amenorrhea risk of doxorubicin and cyclophosphamide. Patients treated with tamoxifen in addition to adjuvant chemotherapy, on the other hand, may have a decreased chance of recovering their regular menses.[7-9]
This wide range of therapy-induced amenorrhea points to the need for well-controlled randomized studies when assessing the risk of therapy-induced amenorrhea, and the potential benefits of interventions to preserve fertility and protect against ovarian damage during adjuvant therapy.
It further outlines the need for biomarkers to assess the individual ovarian reserve at time of diagnosis, to facilitate a personalized calculation of the risk imposed by the cancer therapy.
Predictors of ovarian damage and permanent therapy-induced amenorrhea
FSH and inhibin. Follicle-stimulating hormone (FSH) levels and inhibin A and B levels have been used in many studies to assess and predict therapy-induced ovarian failure.[10,11] However, the fluctuations of these markers between and within cycles render them less predictable, and often both high FSH levels and low inhibin levels after chemotherapy are reversible over time and revert to pretreatment levels when menses recover.[12-14]
Anti-Mllerian hormone. Recent studies have suggested that anti-Mllerian hormone (AMH) levels may be predictive of ovarian reserve and the response to ovarian stimulation. AMH belongs to the transforming growth factor beta (TGF-β) family and is expressed in the ovarian granulosa cell of antral follicles but not in larger developing follicles.[15,16] In contrast to FSH and inhibin levels, AMH does not fluctuate during the menstrual cycle and should not be greatly influenced by the temporary cessation of menses. Several small studies have pointed to the value of AMH as a more reliable predictive marker of permanent chemotherapy-induced amenorrhea.[17-20] Assessment of AMH levels should therefore be incorporated in future studies evaluating interventions to preserve fertility in patients undergoing chemotherapy.
Antral follicle count. In addition to laboratory studies of levels of FSH, inhibin, and AMH, sonographic assessment of antral follicle counts (AFC) have recently been found to be predictive of ovarian reserve and predictors of impending menopause.[21-24] A small study suggested that the assessment of both AMH and AFC were most reliably linked to a positive response to letrozole for ovarian stimulation and the successful embryo/oocyte preservation in patients with breast cancer.[25]
It is hoped that using this approach in addition to assessing levels of AMH, inhibin, and FSH will increase the value of any of these markers in predicting the likelihood of permanent ovarian damage induced by chemotherapy in individual patients.
Benefits of Ovarian Suppression
Several studies have shown that ovarian suppression is an effective means of adjuvant therapy. Several early trials have shown that ovarian suppression is comparable to or better than adjuvant chemotherapy with cyclophosphamide, methotrexate, and fluorouracil (CMF).[26-30]
TABLE 1
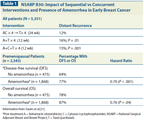
NSABP B30: Impact of Sequential vs Concurrent Interventions and Presence of Amenorrhea in Early Breast Cancer
The quest to preserve ovarian function and retain fertility has recently come under increasing debate, as a large randomized study has shown a considerable breast cancer–specific and overall survival benefit in younger women who did not recover their menses after adjuvant chemotherapy (Table 1).
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B30 trial evaluated the benefits of sequential vs concurrent docetaxel. A secondary aim of this trial, which included 5,351 women, was to determine the effects of prolonged amenorrhea on outcomes. The trial included three arms (Table 1) and found that women with persistent amenorrhea had longer disease-free and overall survival.[31] Surprisingly, this trial also suggested a benefit of amenorrhea in patients with hormone receptor–negative tumors. Similarly, a 12-year, late reassessment of the International Breast Cancer Study Group Trial VIII suggested that CMF followed by goserelin (Zoladex), which resulted in more pronounced and prolonged ovarian suppression, is superior to either modality alone.[29] Several smaller trials and retrospective analyses have evaluated the benefits of adjuvant chemotherapy in women with prolonged amenorrhea vs those who had early resumption of menstruation. These findings are likely prompting the reassessment of several studies in which amenorrhea was a secondary endpoint.
Further investigation has suggested very low rates of breast cancer recurrence in young premenopausal women receiving hormonal therapy with ovarian suppression and endocrine therapy in the absence of chemotherapy. A study of 1,803 patients receiving goserelin and tamoxifen or anastrozole showed disease-free survival rates of 93% in the tamoxifen group and 92% in the anastrozole group after 48 months follow-up.[32]
TABLE 2
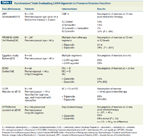
Randomized Trials Evaluating LHRH Agonists to Preserve Ovarian Function
Interventions for fertility preservation in women undergoing chemotherapy
Luteinizing hormone-releasing hormone (LHRH) agonists. Initial reports suggested considerable benefits of LHRH agonists in protecting the ovaries from chemotherapy-induced damage. However, the data showed wide ranges of reported amenorrhea, due at least in part to the highly variable chemotherapy doses and durations, and the absence of stratification by age and treatment with hormone therapy in nonrandomized trials. Table 2 summarizes the six randomized trials evaluating agonists of LHRH (which is also known as gonadotropin-releasing hormone [GnRH]) to preserve ovarian function. In addition, another large multi-institutional trial in women with ER-negative tumors is ongoing: the Southwest Oncology Group (SWOG) 0230 trial, also called the Prevention Of Early Menopause Study (POEMS). Three of the six trials suggest a benefit from LHRH agonists, whereas three trials do not. The differences may be due in part to the shorter follow-up in the positive Egyptian trial by Badawy et al[33] and in PROMISE-GIM6 (Prevention of Menopause Induced by Chemotherapy: A Study in Early Breast Cancer Patients–Gruppo Italiano Mammella 6),[34] in which follow-up was only 6 months and 12 months, respectively. The amenorrhea rates induced by CMF followed by 2 years of endocrine therapy in the ZIPP (Zoladex In Premenopausal Patients) trial are considerably higher than those seen in trials assessing newer regimens, which may be influenced by the high median age of the patients (45 years), with premenopausal women up to the age of 54 enrolled into the trial.[31]
In the three randomized trials with longer follow-up, the benefits of LHRH agonists were no longer seen. In OPTION (Ovarian Protection Trial In Premenopausal Breast Cancer Patients), patients on goserelin actually took longer to recover their menses, but the results should be evaluated with caution, as the final results for the entire 227 patients have not been presented. The three negative trials further differ from the positive trials. Two of the trials were conducted in ER-negative patients only, and the third trial stratified patients by ER status, thus eliminating the confounding effects of hormonal therapy with tamoxifen. Of note, all of the patients who did not resume their menses in the Community Clinical Oncology Program (CCOP) trial led by our group were on tamoxifen.[14] Furthermore, the trials without benefit from LHRH agonists showed amenorrhea rates in the control arms that were much lower than those reported by Badawy et al and Del Mastro et al.[33,34] The amenorrhea rates in the control groups of the ZORO (Zoladex Rescue of Ovarian Function), OPTION, and CCOP studies are comparable with those reported from more recent studies, if the type of regimen, duration of therapy, and age of the patient are taken into consideration. The permanent amenorrhea rates for women under the age of 40 are reported to be around 15%.[7] In the setting of low risk of developing amenorrhea, the benefits of LHRH agonists may indeed be more difficult to assess.
Unfortunately, none of the trials has pointed to reliable individual biomarkers to help predict who is at risk of developing permanent amenorrhea. It has to be further recognized that the resumption of menses does not guarantee the ability to conceive.
Assisted reproductive technology. The high success rate of ART should now be an integral part of the discussion about fertility preservation with women who are undergoing chemotherapy. Recent reports have suggested that fecundity with ART may be similar to natural fecundity in non-cancer patients, and an estimated 15 out of 1,000 live births involve ART. Until recently, a major hurdle in embryo cryopreservation in women with ER-positive breast cancer has been the concern over artificially creating high estrogen levels during ovarian stimulation. Furthermore, perceived time and cost constraints and low estimated success rates in ART have further limited the referral for consideration of ART in breast cancer patients.
However, recent studies have suggested that ovarian stimulation can be safely accomplished with aromatase inhibitors or tamoxifen, preventing high estradiol peaks during ovarian stimulation.[35] Using high doses of tamoxifen (60 mg/d) or letrozole (5 mg/d) with low-dose follicle-stimulating hormone resulted in sufficient numbers of mature oocytes to warrant harvest, fertilization, and embryo cryopreservation. The successful harvest of oocytes can be accomplished within one menstrual cycle and is feasible prior to initiation of chemotherapy. However, embryo cryopreservation requires that the patient have a partner at the time of harvest, or donor sperm are used. Recent advances in ART, however, now also allow the cryopreservation of oocytes not requiring a partner at the time of oocyte harvest and freezing. While the success rates of delayed in vitro fertilization (IVF) with frozen oocytes are lower compared with IVF with unfrozen oocytes,[36] this method still offers women without a partner at the time of diagnosis an option for a later pregnancy with IVF.
The initiation of ovarian stimulation and oocyte harvesting typically requires one or more menstrual cycles, therefore referral to the fertility clinic should occur as early as possible in the patient’s cycle. In most patients, chemotherapy does not start until 2 to 12 weeks after surgery. A referral to the fertility specialist and a possible intervention should be feasible. We have found that most hurdles are vastly reduced when an interdisciplinary collaboration is already in place.
Delayed pregnancy. Given the recent recommendation of prolonged hormonal therapy in the treatment of breast cancer, subsequent pregnancy may often be delayed for several years. Both healthcare professionals and patients should feel reassured that the delay of pregnancy may not be detrimental when donor oocytes or frozen embryos or oocytes are used. With regard to pregnancy, only the age of the oocytes but not the age of the uterus appears to be a factor in the ability to conceive. In fact, recent data suggest that when using the non-donor oocytes, the conservative rate for live births resulting from ART is strongly linked to the age of the patient at time of harvest. A conservative estimate for live birth following IVF with non-donor oocytes in women 30 years of age and younger was 63%; it was 19% at age 42 and only 7% in women over 43 years of age. The chance of a live birth when donor oocytes are used, however, was estimated to be 60% to 80%, as age, ovarian reserve, and ovarian function are no longer limiting factors.[37] The encouraging success rates in embryo and oocyte cryopreservation allow the delay of pregnancy until the woman has completed her breast cancer therapy or can more safely interrupt hormonal therapy.
The success rates of offered interventions in each fertility clinic have been published annually since 1992, by the Centers for Disease Control and Prevention (CDC) under the “The Fertility Clinic Success Rate and Certification Act (FCSRCA),” and provide a valuable referral resource to healthcare professionals and patients.[38]
Preservation of Fertility in BRCA Mutation Carriers
The decision to retain fertility is particularly challenging when caring for women who carry BRCA1 or BRCA2 mutations, in whom removal of the ovaries and fallopian tubes has been shown to significantly impact breast cancer–specific and overall survival. Several reports allude to impaired fertility in mutation carriers and the possibility for these women to undergo menopause sooner than noncarriers.[39-42] However, in a large study of 2,254 BRCA carriers and 764 noncarrier controls from the same family, no clear impact of the BRCA gene mutation on fertility was found in patients at a younger age. Little is known about whether ovarian tissue in mutation carriers is more susceptible to chemotherapy damage, and if so, by what mechanism.
Given the increased risk of ovarian cancer in this population and the clearly established benefits of oophorectomy with respect to breast cancer survival, protecting against ovarian damage has to be weighed against the benefits of risk-reducing bilateral salpingo-oophorectomies.
Patients often struggle with the possibility of passing on to their offspring a mutation that conveys a higher cancer risk. Recent advances in pre-implantation diagnosis make it possible to reduce the chance of passing on the mutated gene. With such options, patients may find it more desirable to protect their ovaries, and to pursue fertility preservation at a younger age prior to undergoing risk-reducing oophorectomies after completion of childbearing.[43] The complexity of fertility preservation in this patient population should prompt a timely referral to well-informed experts in the field.
Current Practice for Patients of Childbearing Age With Breast Cancer
A survey performed by our group in 2007 suggested that even among healthcare professionals who are medical and surgical oncologists, an in-depth discussion about fertility did not occur consistently. Although physicians discussed the risk of cancer therapy on fertility, patients were not referred to a reproductive center with expertise in cancer owing to perceived risks, cost, and time constraints.[44] We found that, at the time of diagnosis, neither patients nor healthcare professionals considered fertility a high priority, in light of more pressing concerns. However, the relevance of fertility preservation shifted at later time points, when mortality no longer appeared to be an immediate concern. Not addressing fertility issues and considering potential options to preserve fertility was associated with considerable regret.
These findings were more formally explored in a larger comprehensive study evaluating patterns of pretreatment fertility counseling and referrals. Although retrospective, this study surveyed more than 1,000 patients. Loss of fertility resulted in a considerable detriment to patients’ quality of life. Counseling about fertility loss and discussion of fertility-preservation options resulted in better long-term acceptance of fertility loss.[31,45,46] The group further reported that only 5% of patients whose fertility was at risk were counseled by a reproductive endocrinologist, and even fewer patients pursued fertility preservation.
Conclusion
Several studies have suggested that the option to preserve fertility is a very important factor for women diagnosed with cancer during their childbearing years. Despite many recent advances, however, there is still uncertainty about how to optimally predict ovarian reserve pre- and post-therapy, and how to choose and incorporate the most successful interventions. In nonrandomized studies and three recently reported randomized studies, the use of LHRH agonists has been proposed to protect against ovarian damage during chemotherapy. However, data from three further randomized studies have not shown a benefit from LHRH agonists when newer chemotherapy regimens with shorter duration were used, and patients were stratified for hormone receptor expression and tamoxifen use. These studies also show substantially lower amenorrhea rates, and often, upon longer follow-up, resumption of menses in over 80% of women. Adding to the complexity are recent studies reporting improved breast cancer outcomes in women receiving regimens that cause prolonged amenorrhea, and non-chemotherapy regimens with prolonged ovarian suppression have high disease-free survival rates, comparable to those seen with chemotherapy. Emerging data on the benefits of hormonal therapy beyond 5 years will have to be weighed against the natural decrease of ovarian reserve occurring in patients (with the exception of women who are very young at diagnosis) over the course of 10 years. Furthermore, the recognition of a higher likelihood of late recurrences in women with hormone receptor–positive breast cancer may render the timing of pregnancy in these women particularly challenging. In times of rapid advances in reproductive endocrinology allowing preservation of embryos and oocytes, we feel that alternative strategies should be considered in addition rather than solely relying on LHRH agonists to preserve ovarian function. All women who have not completed their family planning should be offered a comprehensive consultation involving breast surgeons, medical oncologists, and reproductive endocrinologists as early as possible in the diagnosis.
Financial Disclosure:The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29.
2. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2011. Natl Vital Stat Rep. 2011;61:1-20. Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_05.pdf
3. Faddy MJ, Gosden RG, Gougeon A, et al. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342-6.
4. Luborsky JL, Meyer P, Sowers MF, et al. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18:199-206.
5. Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865-74.
6. Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769-79.
7. Fornier MN, Modi S, Panageas KS, et al. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer. 2005;104:1575-9.
8. Davis AL, Klitus M, Mintzer DM. Chemotherapy-induced amenorrhea from adjuvant breast cancer treatment: the effect of the addition of taxanes. Clin Breast Cancer. 2005;6:421-4.
9. Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer. 2010;116:3102-11.
10. Burkhardt N, Juckstock J, Kuhn C, et al. Inhibin A is down-regulated during chemotherapy in patients with breast cancer. Anticancer Res. 2010;30:4563-6.
11. Su HI, Sammel MD, Green J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592-9.
12. van Beek RD, van den Heuvel-Eibrink MM, Laven JS, et al. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin’s lymphoma during childhood. J Clin Endocrinol Metab. 2007;92:3869-74.
13. Boyers SP, Luborsky JL, DeCherney AH. Usefulness of serial measurements of serum follicle stimulating hormone, luteinizing hormone and estradiol in patients with premature ovarian failure. Fertil Steril. 1988;50:408-12.
14. Munster PN, Moore AP, Ismail-Khan R, et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:533-8.
15. Themmen AP. Anti-Mullerian hormone: its role in follicular growth initiation and survival and as an ovarian reserve marker. J Natl Cancer Inst Monogr. 2005;18-21.
16. Loh JS, Maheshwari A. Anti-Mullerian hormone--is it a crystal ball for predicting ovarian ageing? Hum Reprod. 2011;26:2925-32.
17. Anderson RA, Cameron DA. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336-43.
18. Decanter C, Morschhauser F, Pigny P, et al. Anti-Mullerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online. 2010;20:280-5.
19. Lie Fong S, Laven JS, Hakvoort-Cammel FG, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Mullerian hormone. Hum Reprod. 2009;24:982-90.
20. Bath LE, Wallace WH, Shaw MP, et al. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18:2368-74.
21. Mutlu MF, Erdem M, Erdem A, et al. Antral follicle count determines poor ovarian response better than anti-mullerian hormone but age is the only predictor for live birth in in vitro fertilization cycles. J Assist Reprod Genet. 2013 Mar 19. [Epub ahead of print]
22. Wellons MF, Bates GW, Schreiner PJ, et al. Antral follicle count predicts natural menopause in a population-based sample: the Coronary Artery Risk Development in Young Adults Women’s Study. Menopause. 2013 Feb 15. [Epub ahead of print]
23. Bentzen JG, Forman JL, Larsen EC, et al. Maternal menopause as a predictor of anti-Mullerian hormone level and antral follicle count in daughters during reproductive age. Hum Reprod. 2013;28:247-55.
24. Bonilla-Musoles F, Castillo JC, Caballero O, et al. Predicting ovarian reserve and reproductive outcome using antimullerian hormone (AMH) and antral follicle count (AFC) in patients with previous assisted reproduction technique (ART) failure. Clin Exp Obstet Gynecol. 2012;39:13-8.
25. Lee S, Ozkavukcu S, Heytens E, et al. Anti-Mullerian hormone and antral follicle count as predictors for embryo/oocyte cryopreservation cycle outcomes in breast cancer patients stimulated with letrozole and follicle stimulating hormone. J Assist Reprod Genet. 2011;28:651-6.
26. Kaufmann M, Jonat W, Blamey R, et al. Survival analyses from the ZEBRA study. Goserelin (Zoladex) versus CMF in premenopausal women with node-positive breast cancer. Eur J Cancer. 2003;39:1711-7.
27. Jonat W, Kaufmann M, Sauerbrei W, et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The Zoladex Early Breast Cancer Research Association Study. J Clin Oncol. 2002;20:4628-35.
28. Jakesz R, Hausmaninger H, Kubista E, et al. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer--Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002;20:4621-7.
29. Karlsson P, Sun Z, Braun D, et al. Long-term results of International Breast Cancer Study Group Trial VIII: adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node-negative breast cancer. Ann Oncol. 2011;22:2216-26.
30. Castiglione-Gertsch M, O’Neill A, Price KN, et al. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2003;95:1833-46.
31. Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053-65.
32. Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679-91.
33. Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91:694-7.
34. Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306:269-76.
35. Oktay K, Buyuk E, Libertella N, et al. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347-53.
36. Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86:70-80.
37. Luke B, Brown MB, Wantman E, et al. Cumulative birth rates with linked assisted reproductive technology cycles. N Engl J Med. 2012;366:2483-91.
38. Fertility Clinic Success Rate and Certification Act (FCSRCA). Available from: http://www.cdc.gov/art/artreports.htm
39. Finch A, Valentini A, Greenblatt E, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril. 2013;99:1724-8.
40. Moslehi R, Singh R, Lessner L, Friedman JM. Impact of BRCA mutations on female fertility and offspring sex ratio. Am J Hum Biol. 2010;22:201-5.
41. Pal T, Keefe D, Sun P, Narod SA. Fertility in women with BRCA mutations: a case-control study. Fertil Steril. 2010;93:1805-8.
42. Lin WT, Beattie M, Chen LM, et al. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer. 2013;119:1652-9.
43. Sagi M, Weinberg N, Eilat A, et al. Preimplantation genetic diagnosis for BRCA1/2--a novel clinical experience. Prenat Diagn. 2009;29:508-13.
44. Quinn GP, Vadaparampil ST, Gwede CK, et al. Discussion of fertility preservation with newly diagnosed patients: oncologists’ views. J Cancer Surviv. 2007;1:146-55.
45. Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118:1710-7.
46. Letourneau JM, Melisko ME, Cedars MI, Rosen MP. A changing perspective: improving access to fertility preservation. Nature Rev Clin Oncol. 2011;8:56-60.
47. Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat. 2009;117:561-7.
48. Gerber B, von Minckwitz G, Stehle H, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;29:2334-41.
49. Leonard RC, Adamson D, Anderson R, et al. The OPTION trial of adjuvant ovarian protection by goserelin in adjuvant chemotherapy for early breast cancer. Cancer Res. 2010;24(suppl 2):70.