Tailored Therapy in Diffuse Gliomas: Using Molecular Classifiers to Optimize Clinical Management
We review the current data regarding the prognostic and predictive value of IDH mutation and 1p/19q codeletion in gliomas. We also discuss possible management algorithms using these biomarkers to tailor surgical and adjuvant therapy for specific diffuse gliomas.
Diffuse gliomas are the most common primary malignant brain tumors in adults and continue to be almost universally fatal. Nevertheless, a striking variability in outcome has long been observed, with a subset of patients having prolonged survival. Recent molecular discoveries have provided new insights into gliomagenesis and have enhanced clinical subclassification of gliomas. Mutations in the isocitrate dehydrogenase (IDH) genes occur frequently in low-grade astrocytomas and oligodendrogliomas (World Health Organization [WHO] grade II), and in higher-grade gliomas (WHO grades III and IV) that arise after malignant progression of low-grade tumors. IDH mutation has an established role as a favorable prognostic marker; however, the utility of IDH mutation in guiding treatment is still under investigation. A subset of IDH-mutant tumors, predominantly oligodendrogliomas, also harbor codeletion of chromosomes 1p and 19q, a feature that predicts responsiveness to chemotherapy. Here, we review the current data regarding the prognostic and predictive value of IDH mutation and 1p/19q codeletion in gliomas. We also discuss possible management algorithms using these biomarkers to tailor surgical and adjuvant therapy for specific diffuse gliomas. Ultimately, understanding the natural history of glioma subtypes and the predictive value of genetic markers may maximize survival and minimize treatment morbidity.
Introduction
Diffuse gliomas continue to be difficult malignancies to manage despite the recent discovery of strong prognostic molecular markers. Gliomas account for 30% of the incidence of primary central nervous system (CNS) tumors, which is 26 per 100,000 for adults ≥ 20 years old.[1] The World Health Organization (WHO) grades gliomas on a scale of I to IV, with higher grades corresponding to more malignant features. Pathologically, gliomas are divided into astrocytic (75%) or oligodendroglial tumors (25%), depending on the primary histology, with rare cases of mixed histology.[2] WHO grade II diffuse gliomas (also known as low-grade gliomas) and grade III (anaplastic) gliomas are less common than grade IV gliomas (glioblastomas). Glioblastoma is the most common malignant primary brain tumor in adults and accounts for > 30% of all gliomas. Primary glioblastomas are defined as tumors that are discovered de novo, whereas secondary GBMs are tumors that arise after malignant progression via transformation of a lower-grade glioma.[1]
In young adults (aged 20 to 34 years), gliomas account for 32% of primary brain tumors.[1] Treatment of this population poses a particular set of challenges. It has long been observed that these patients have improved survival compared with older patients with gliomas of identical histology; however, it remains uncertain whether these patients should be treated differently. The recent discovery of recurrent somatic mutations in the isocitrate dehydrogenase 1 (IDH1) and IDH2 genes in gliomas has provided substantial insight into the improved outcomes seen in subsets of patients.[3-5] IDH mutations correlate with younger age at diagnosis, accounting for most of the prognostic effect of age.[6] Furthermore, IDH mutations are also independently associated with improved survival.[5,7]
Although knowledge of the biology and natural history of IDH-mutant gliomas is increasing rapidly, how to incorporate this powerful prognostic marker into clinical management remains unresolved. Here, we review the epidemiology and pathophysiology of IDH-mutant gliomas, the association of IDH mutation with other molecular markers, and the value of IDH mutation as a prognostic and possibly predictive marker. Finally, we will discuss how our understanding of molecular prognostic factors could be used to stratify therapeutic decisions for patients with malignant gliomas.
Epidemiology and Diagnosis
Mutations in the IDH genes were discovered in 2008 as a result of genome-wide sequencing efforts in glioblastoma. In the initial report, recurrent mutations in the R132 position of the IDH1 gene were observed in 12% of glioblastoma cases, and these mutations were enriched in patients with secondary glioblastoma.[3] Further investigation in diverse primary brain tumors identified recurrent mutations in IDH1 or in the analogous amino acid position R172 of the related gene IDH2 in 55% to 80% of WHO grade II and III diffuse gliomas. Conversely, IDH mutations were found in only 5% to 10% of primary glioblastomas and in < 10% of other primary CNS tumors, such as pilocytic astrocytomas, pediatric glioblastomas, ependymomas, and meningiomas.[3,4,8-11] In addition to gliomas, IDH mutations are frequently observed in acute myeloid leukemia,[12] in intrahepatic cholangiocarcinoma,[13] and in chondrosarcoma.[14]
As suggested above, patients with IDH-mutated gliomas have a distinct clinical phenotype. They are significantly younger than those with IDH−wild-type tumors across all tumor grades.[6] The incidence peaks in patients in their third decade, and mutations are rarely seen in the pediatric or elderly population.[15] High-grade IDH-mutant gliomas also have distinct radiographic characteristics. There are predilections for frontal lobe location, larger size at diagnosis, more frequent nonenhancing tumor component, less contrast enhancement, and reduced necrotic-appearing areas, as well as for more prevalent cystic and diffuse components.[6,15] These features are similar to the classic radiographic pattern associated with lower-grade diffuse gliomas.
IDH mutations are strongly associated with other molecular markers of improved survival, including O6-methylguanine-DNA methyltransferase (MGMT) promoter DNA methylation and cytosine-phosphate-guanine (CpG) island hypermethylator phenotype (G-CIMP).[7,15,16] Furthermore, deep genomic sequencing analyses have subclassified IDH-mutant tumors based on the tightly clustered association of IDH mutation with other genetic alterations. IDH mutation is frequently observed with TP53 and alpha thalassemia/mental retardation syndrome X-linked (ATRX) mutations in astrocytic tumors, and with deletion of 1p and 19q (1p/19q codeletion) and mutations in the homologue of the Drosophila capicua gene (CIC) and the promoter of the telomerase reverse transcriptase gene (TERT) in pure oligodendrogliomas.[7,15,17-20]
Perhaps the most striking clinical feature of patients with IDH-mutated gliomas, however, is the improved survival seen across all glioma types compared with patients with IDH−wild-type gliomas. Despite the relatively recent discovery of IDH mutations in gliomas, the prognostic data have come rapidly from retrospective analyses of tissue banks from clinical trials. IDH1 and IDH2 status was determined in subsets of patients from two large prospective, randomized trials of newly diagnosed anaplastic gliomas (the German NOA-04 and the European Organisation for Research and Treatment of Cancer [EORTC] 26951 trials), and after adjusting for other prognostic factors-such as age, histology, grade, extent of resection, MGMT promoter methylation, and 1p/19q codeletion-IDH1 mutation remained a strongly significant independent prognostic factor.[5,7,21,22] In the NOA-04 analysis, IDH1 mutation was the dominant prognostic factor. Furthermore, IDH analysis of glioblastomas from the German Glioma Network[23] and of anaplastic astrocytomas from the NOA-04 trial[22] indicated that the median survival for IDH−wild-type anaplastic astrocytomas was worse than that of IDH-mutated glioblastoma, suggesting that IDH mutational status may be considered as important a prognostic marker as histology in grade III and IV astrocytomas.[5]
The rapid diagnostic assessment of IDH status has been greatly facilitated by the development of an antibody directed against the most prevalent IDH-mutant isoform (IDH1 R132H substitution-which accounts for approximately 85% of all IDH mutations in gliomas[4,6]), as this antibody has become widely available and has been adopted in many clinical diagnostic laboratories. Compared with sequencing, the sensitivity and specificity of the IDH1 R132H antibody to detect all variants of IDH1 by immunohistochemistry (IHC) have been reported to be 94% and 100%, respectively.[24] There may be higher inter-laboratory agreement for IHC than for sequencing. One study demonstrated 100% concordance for IDH1 R132H IHC results across laboratories in a test set of gliomas, whereas several laboratories had inconsistent results using sequencing.[21] In addition to facilitating the genotyping of IDH in diffuse gliomas, IHC for IDH1 status has improved the accuracy of histopathological diagnosis in cases with limited sample available, thereby aiding in distinguishing between diffuse gliomas and pilocytic astrocytomas, ependymomas, and nonneoplastic reactive gliosis.[24-26]
The Pathophysiology of IDH Mutations
Mutations in IDH are nearly all heterozygous missense somatic mutations at codon 132 (IDH1) or 172 (IDH2), which are important structural residues for enzymatic function and ligand binding. Upwards of 90% of IDH1 mutations are single-base transition substitutions from G to A, resulting in an arginine to histidine substitution (R132H), with other mutations occurring less frequently. IDH2 mutations are much less common (~3%) and are associated with oligodendroglial histology.[4,6,10]
IDH is a catalytic enzyme with at least three isoforms-IDH1, IDH2, and IDH3. IDH3 is the canonical nicotinamide adenine dinucleotide (NAD+)-dependent enzyme involved in the Krebs cycle, and mutations in IDH3 have not been observed in gliomas. The normal function of IDH1, located in the cytoplasm, is to catalyze the oxidative decarboxylation of isocitrate into alpha-ketoglutarate (alpha-KG) and nicotinamide adenine dinucleotide phosphate (NADPH). These metabolites are then thought to be involved in cholesterol and lipid synthesis. The identical reaction is carried out by IDH2 in the mitochondria.[27] The mutated IDH enzyme loses the ability to generate alpha-KG and gains the ability to convert alpha-KG to 2-hydroxyglutarate (2-HG),[28] which then accumulates to significant levels in gliomas.[29]
Due to the exceedingly high levels detected in glioma tissue-up to one to two orders of magnitude higher than normal-2-HG has been proposed as a novel oncometabolite. However, its exact mechanistic role in gliomagenesis is still under active investigation. Emerging data suggest that 2-HG exerts its oncogenic effect by competitively inhibiting 2-oxoglutarate (2-OG)-dependent enzymes, such as hypoxia-inducible factor prolyl hydroxylases, methyl-cytosine hydroxylases (which regulate DNA methylation), and histone demethylases,[30-32] which result in global histone and DNA hypermethylation and in induction of G-CIMP.[15,32,33] Some of these effects may also result in accumulation of hypoxia-inducible factor (HIF)-1α and increased angiogenesis.[34,35]
As mentioned above, other genetic alterations have recently been found to cluster tightly with IDH mutations in gliomas, raising the possibility that the mechanistic basis of prognostic and predictive impact may, in fact, be due to these additional alterations. These alterations therefore present the opportunity for further stratification. Patients with concurrent IDH, p53, and ATRX mutations have an “intermediate” median overall survival of approximately 5 years. Mutations in the CIC gene on chromosome 19q and in the far upstream binding protein 1 (FUBP1) gene on chromosome 1p are associated with classical histologic oligodendrogliomas. As would be expected, CIC mutations strongly correlate with 1p/19q codeletion and, when present with IDH mutations, are associated with a median overall survival of 8 or more years. If none of these alterations are present, the median overall survival is approximately 1 year, regardless of tumor grade,[19] essentially mirroring the natural history of classical glioblastoma.
Prognostic and Predictive Implications
Recently mature data from the large, prospective, randomized Radiation Therapy Oncology Group (RTOG) and EORTC trials investigating the role of chemotherapy (procarbazine, lomustine [CeeNU], and vincristine [PCV regimen]) in addition to radiation in newly diagnosed anaplastic oligodendroglial tumors provide intriguing insight into prognostic and predictive markers for anaplastic gliomas. An estimated 25% to 50% of anaplastic oligodendrogliomas and oligoastrocytomas harbor 1p/19q codeletion and associated IDH mutations. Although these trials opened to accrual in the 1990s, prior to the discovery of IDH1 mutation[3] or the prognostic and potentially predictive importance of 1p/19q codeletion,[36] 1p/19q status was determined in 91% of patients in the RTOG study and in 86% in the EORTC study.
Regardless of treatment assignment, in both studies patients with 1p/19q codeletion survived much longer than patients with non-codeleted tumors (eg, overall survival of 123 vs 23 months in the EORTC study), confirming the strong prognostic effect of 1p/19q codeletion.[37,38] In addition, these studies report data suggesting that 1p/19q codeletion predicts sensitivity to PCV chemotherapy. In the RTOG study, patients with codeletion who received PCV in addition to radiation at diagnosis lived much longer than those treated with radiation alone (median overall survival of 14.7 vs 7.3 years, P = .03). Conversely, there was no difference in the survival of patients with non-codeleted tumors in the different treatment arms. In the EORTC study, the median survival of patients with 1p/19q codeleted tumors who received radiation and PCV had not yet been reached, compared with 112 months for patients with codeleted tumors treated with radiation alone. Of note, this difference was a trend for extended survival in the chemotherapy group (P = .059). As with the RTOG study, in the non-codeleted subset, there was no difference in survival between treatment arms.[7,37]
The prognostic impact of IDH mutation was also confirmed in both studies. The EORTC study retrospectively evaluated IDH1 status in 179 of 368 enrolled patients, and IDH mutation was found to be an independent prognostic indicator, with median overall survivals of 8.4 years for patients with IDH-mutated tumors vs 1.4 years for patients with IDH−wild-type tumors. IDH mutation was confirmed to be an important prognostic factor in the RTOG study as well. However, the question of whether IDH mutation itself predicts sensitivity to chemotherapy remains unresolved. In the EORTC study, IDH-mutant tumors seemed to derive more benefit from PCV chemotherapy; however, tests for interactions with chemotherapy were not statistically significant.[7,37]
In NOA-04, a randomized phase III trial of radiation vs chemotherapy (either PCV or the currently more widely used alkylating agent temozolomide [Temodar]) in anaplastic gliomas, IDH1 mutation was retrospectively reviewed in a subset of patients and was found to be associated with longer time to treatment failure but was not predictive of response to chemotherapy.[22] A follow-up study demonstrated that IDH1 mutation was an independent prognostic factor for overall survival, and was in fact the most prominent prognostic factor for survival.[5]
As a result of these retrospective analyses, the National Comprehensive Cancer Network (NCCN) guidelines now recommend the use of chemotherapy with radiation for treatment of anaplastic gliomas harboring a 1p/19q codeletion.[39]
In low-grade gliomas, the predictive value of IDH mutations with regard to radiation or chemotherapy has yet to be determined. One retrospective study of 84 patients with low-grade gliomas who were treated with temozolomide upfront after surgical resection reported that IDH-mutant tumors had higher rates of radiographic responses; the response rate was highest in patients with both IDH1 mutation and 1p/19q codeletion.[40] However, other retrospective studies of low-grade astrocytomas failed to correlate IDH1 mutation with temozolomide response.[41,42]
Given the mounting evidence that 1p/19q codeletion conveys sensitivity to chemotherapy, two large, multicenter randomized trials will address the issues of temozolomide efficacy and chemosensitivity in 1p/19q codeleted and non-codeleted anaplastic tumors. The CODEL trial (NCT00887146), a phase III study currently under review by the RTOG, is considering randomization of patients with newly diagnosed anaplastic gliomas with 1p/19q codeletion to either PCV, temozolomide, or combined chemotherapy and radiation. The specific regimens in each arm remain to be determined. For patients with newly diagnosed anaplastic gliomas without 1p/19q codeletion, the counterpart trial, CATNON (NCT00626990), is currently randomly assigning patients to one of four arms: radiation alone; radiation with concurrent daily temozolomide without further adjuvant therapy; radiation alone, followed by 12 cycles of adjuvant temozolomide; and finally, concurrent daily chemoradiation followed by 12 cycles of temozolomide. In this study, patients will be stratified on the basis of MGMT status.
Tailoring Treatment
While the discovery of the prognostic significance of IDH mutation, like that of prior molecular biomarkers 1p/19q codeletion and MGMT promoter methylation, has been of great assistance to patient counseling and clinical trial stratification, the lack of prospective data on the impact of IDH mutation on therapeutic response has perhaps added to the complexity of treatment decisions. The core of this challenge is that prognosis itself provides little information on the efficacy of individual treatment modalities in each molecular subtype.
Should IDH-mutant cases be treated aggressively with multimodal therapy upfront, accepting the risks of short-term and delayed morbidity with radiation and chemotherapy, in order to achieve the potential goal of delaying recurrence for a decade or more? Conversely, does the slower growth rate of IDH-mutant gliomas allow for a more conservative approach, in which adjuvant therapy is delayed and toxicity is obviated until recurrence? Our growing understanding of the prognostic and predictive effects of IDH mutation and 1p/19q codeletion is beginning to shed light on how to apply these markers in therapeutic decision-making, and is highlighting areas of controversy. Here, we will illustrate, using several clinical scenarios, how these markers may be used to tailor approaches to both surgical intervention and adjuvant treatment.
Case #1
FIGURE 1
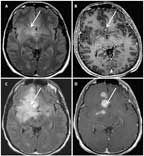
MRI of a 41-Year-Old Man With an Anaplastic Astrocytoma, IDH–Wild- Type
A 41-year-old man presented with a generalized seizure. MRI revealed an extensive, diffusely infiltrative, largely nonenhancing lesion within the right frontal and temporal lobes (Figures 1A and 1B). A biopsy of the right frontal lobe demonstrated an anaplastic astrocytoma, IDH1−wild-type. The patient was treated with concurrent radiation and temozolomide followed by adjuvant temozolomide, per standard protocol for glioblastoma. After 1 cycle of temozolomide, his MRI showed progressive disease (Figures 1C and 1D), and rebiopsy demonstrated transformation to glioblastoma.
Case #2
FIGURE 2
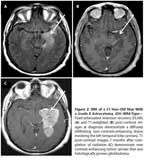
MRI of a 51-Year-Old Man With a Grade II Astrocytoma,
IDH
–Wild-Type
A 51-year-old man presented with complex partial seizures, and MRI demonstrated a nonenhancing, infiltrating lesion involving the anterior and medial left temporal lobe, insula, and thalamus (Figures 2A and 2B). A biopsy was performed, and pathology was interpreted as grade II infiltrating astrocytoma. The patient was treated adjuvantly with involved-field radiation; however, 10 months after diagnosis, an MRI revealed new enhancement throughout the left temporal lobe causing significant mass effect (Figure 2C). Subtotal resection and biopsy demonstrated transformation to GBM. Retrospective analysis revealed this tumor to be IDH1−wild-type. He was treated with repeat radiation (2880 cGy) and concomitant temozolomide; however, he died a few months later. Overall survival from diagnosis of grade II astrocytoma was 15 months.
Impact of molecular classifiers. The absence of IDH mutation in an anaplastic glioma or even a low-grade glioma usually portends poor prognosis (“glioblastoma-like”). Thus, a strong argument can be made to treat aggressively with the combined concurrent chemoradiation regimen used in glioblastoma for this molecular class of tumor.
Unresolved questions. Do IDH−wild-type grade II and III gliomas benefit from the concurrent chemoradiation therapy used for glioblastoma? The results from the ongoing NCT00626990 CATNON anaplastic glioma and NCT00978458 low-grade glioma trials will inform these clinical scenarios.
Case #3
FIGURE 3
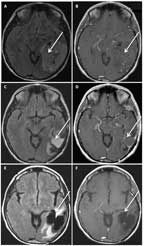
MRI of a 54-Year-Old Woman With an Anaplastic Oligodendroglioma, IDH1- Mutant, 1p/19q Codeleted
A 54-year-old woman presented with several years of progressive cognitive impairment. An MRI revealed a large, 7.7 × 5.6-cm calcified, nonenhancing lesion within the left temporal and parietal lobes (Figures 3A and 3B). Using preoperative speech mapping and awake surgery, a gross total resection of the tumor was achieved (Figures 3C and 3D). Pathology was anaplastic oligodendroglioma. Retrospectively, the patient’s tumor was found to have IDH1 R132H mutation and 1p/19q codeletion. Postoperatively, she received 18 cycles of temozolomide alone. She remained progression-free for over 6 years, when imaging revealed a slowly expanding, nonenhancing asymptomatic lesion. She underwent a second gross total resection, and the pathology remained anaplastic oligodendroglioma. Postoperatively, she was treated with radiation (60 Gy). Currently, 8 years after her diagnosis, she remains radiographically stable (Figures 3E and 3F) and neurologically intact.
Case #4
FIGURE 4
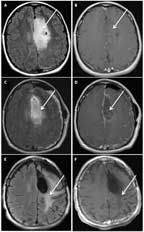
MRI of a 35-Year-Old Woman With a Grade II Oligodendroglioma, IDH1-Mutant, 1p/19q Codeleted
A 35-year-old woman presented with a generalized seizure. An MRI revealed a well-circumscribed, minimally enhancing lesion within the left frontal and parietal lobes (Figures 4A and 4B). The patient underwent gross total resection (Figures 4C and 4D), and pathology revealed a grade II oligodendroglioma. She was treated adjuvantly with 6 cycles of PCV followed by involved-field radiation. She has been progression-free for > 12 years; however, her MRI demonstrated extensive fluid-attenuated inversion recovery (FLAIR) changes consistent with treatment effect (Figures 4E and 4F). During treatment, she developed significant cognitive impairment, with poor attention and short-term memory loss, and these issues have not resolved. She could no longer work as an accountant, and she has remained on long-term disability since her diagnosis. Retrospectively, her tumor was found to be IDH1-mutant, 1p/19q-codeleted.
Impact of molecular classifiers. Careful preoperative planning in Case #3 resulted in gross total resection of the patient’s large tumor, which abutted eloquent areas. The presence of an IDH mutation and 1p/19q codeletion in an anaplastic glioma predicts a substantial survival benefit with the addition of PCV chemotherapy to radiation therapy. The presence of both IDH mutation and 1p/19q codeletion predict a protracted natural history and sensitivity to chemotherapy in addition to radiotherapy.
Case #4 exemplifies the potential risk of morbidity with aggressive postoperative treatment. In contrast to the low-grade tumor in Case #2, this tumor was IDH1-mutant and 1p/19q codeleted, findings that predict prolonged survival. Knowledge of these markers at diagnosis might have justified a more conservative approach in order to obviate toxicity. The EORTC 22845 study of radiation in low-grade astrocytomas and oligodendrogliomas demonstrated no difference in overall survival when administration of radiation was deferred until tumor progression,[43] although some clinical benefit was evident with treatment immediately post-resection, given that progression-free survival was prolonged and seizure frequency lessened. In the RTOG 9802 protocol for low-grade gliomas, patients with small (< 4 cm) oligodendrogliomas that were extensively resected (< 1 cm residual disease) had a progression-free survival of 70% after 5 years of observation-based follow-up.[44]
Unresolved questions. Is adjuvant chemotherapy alone, with deferral of radiation until progression, justified for IDH-mutant, 1p/19q-codeleted anaplastic gliomas? Is the efficacy of temozolomide, which is currently widely used for anaplastic gliomas, equivalent to PCV for 1p/19q-codeleted tumors? Furthermore, does concurrent chemotherapy and radiation, as used in standard glioblastoma protocols, provide additional survival benefit or increased toxicity for 1p/19q-codeleted tumors? The CODEL study will hopefully provide answers to some of these questions; however, it may be a decade or more before survival results are reported. An important focus will be on quality-of-life and neurocognitive outcomes, due to the prolonged anticipated survival of patients with tumors in this molecular group.
For diffuse, low-grade gliomas that are biopsy-proven to be IDH-mutant and 1p/19q-codeleted, can maximal surgical resection be deferred until after neoadjuvant treatment and response,[45,46] with the potential for increased neurologic safety of surgery?
Case #5
FIGURE 5
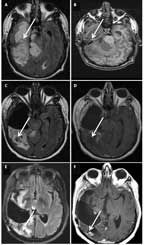
MRI of a 42-Year-Old Man With an Anaplastic Oligoastrocytoma, IDH1-Mutant and 1p/19q-Maintained
A 42-year-old man presented with a headache and visual disturbances. MRI revealed a large, nonenhancing, T1 intrinsically hyperintense, 10.6 × 6.7 × 5.2-cm mass involving the right temporal and occipital lobes, with exophytic components (Figures 5A and 5B). The patient underwent partial resection (Figures 5C and 5D). Pathological and molecular analysis revealed an anaplastic oligoastrocytoma, IDH1-mutant and 1p/19q-maintained. He received 10 cycles of adjuvant temozolomide, and was then observed. Two years later his disease progressed and he was rechallenged with temozolomide, although progression occurred again after 9 cycles. He was then treated with involved-field radiation, and he remained progression-free for another 3 years. His tumor once more recurred, and he underwent repeat resection followed by PCV. After 2 cycles of PCV, his disease progressed again. He is alive 7 years after diagnosis, with mild cognitive impairment and left homonymous hemianopia (Figures 5E and 5F).
Impact of molecular classifiers. The presence of IDH mutation and lack of 1p/19q codeletion in an anaplastic glioma identifies a molecular class of tumors with intermediate survival, for which there are no randomized data to guide treatment decisions. Surgery, radiation, and chemotherapy, either alone or in combination, are all options for this patient population. With regard to surgery, resection of most-or-all enhancing disease is associated with improved survival in anaplastic astrocytomas[47] and glioblastomas.[48,49] However, not all high-grade gliomas are enhancing, and in anaplastic lesions, the survival impact of extent-of-resection of nonenhancing disease is unclear.[47] On the other hand, in low-grade gliomas, the vast majority of which are IDH-mutant, greater extent of resection of nonenhancing disease is clearly associated with improved survival.[50] Given their common molecular lineage, high-grade IDH-mutant gliomas may similarly benefit from a more extensive resection of nonenhancing disease.[51] Therefore, a “second-stage” resection targeting nonenhancing tumor prior to initiation of further adjuvant therapy could be considered for Case #5.
Retrospective data from the EORTC 26951, RTOG 9402, and NOA-04 trials suggest that the survival benefit of chemotherapy was primarily limited to patients with 1p/19q-codeleted tumors.[22,37,38] Consistent with these findings, it is difficult to determine whether there was any benefit from chemotherapy in Case #5. The patient’s protracted course could merely represent the slowly progressive natural history of an IDH-mutant anaplastic glioma. The ongoing CATNON trial, which randomly assigns patients to various radiation and chemotherapy regimens, will hopefully provide guidance for the optimal management of this molecular subclass of IDH-mutant anaplastic gliomas.
These five cases highlight the role that IDH and 1p/19q status can potentially have in tailoring therapy of malignant gliomas; they also highlight areas where controversy exists regarding treatment strategies. We propose an algorithm that incorporates these biomarkers on the basis of the current data (Figure 6). After deciding on the surgical approach, two decision branch points are delineated for the adjuvant therapy of a patient with a diffuse WHO grade II or III glioma: one is dependent on IDH mutation status, and the other is dependent on 1p/19q status.
FIGURE 6
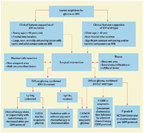
Proposed Algorithm for Tailoring Surgery and Adjuvant Treatment for Suspected Gliomas Based on IDH and 1p/19q Status
While the initial surgical strategy may be straightforward in a low–anesthetic-risk patient with a tumor in a noneloquent location, biopsy could be favored in cases where the clinical data are more equivocal, and molecular stratification could drive treatment decision making. For enhancing lesions that are suspected to be high-grade gliomas, the general goal of surgery is resection of most-or-all of the enhancing component of the disease, while preserving neurologic function and Karnofsky performance status to allow for further therapy.[47-49] For lower-grade lesions (and perhaps IDH-mutant high-grade gliomas), the goal is maximal resection of nonenhancing disease. Ultimately, the key to surgical decision making in the modern molecular era is to allow for a “course correction” as the histologic grading and molecular data become available from the initial procedure (see Figure 6).
In summary, recently discovered genetic markers have increased our understanding of the biology of gliomas, are refining the classification of primary brain tumors, and have identified subsets of patients who have improved outcomes. IDH mutation and 1p/19q codeletion have been validated as robust prognostic markers, with both predicting longer survival, while 1p/19q codeletion is also a predictive biomarker for chemotherapy response. With increasing knowledge of both the prognostic and predictive significance of these markers, their role in tailoring therapy is emerging. Ongoing and future prospective studies will further clarify the role of these markers in the clinical management of malignant gliomas.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Central Brain Tumor Registry of the United States (CBTRUS). CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004-2008. 2012 [updated 2012; cited January 26, 2013]. Available from: http://www.cbtrus.org.
2. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
3. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807-12.
4. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765-73.
5. Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707-18.
6. Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469-74.
7. van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16:1597-604.
8. Balss J, Meyer J, Mueller W, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597-602.
9. Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11:341-7.
10. Kloosterhof NK, Bralten LB, Dubbink HJ, et al. Isocitrate dehydrogenase-1 mutations: a fundamentally new understanding of diffuse glioma? Lancet Oncol. 2011;12:83-91.
11. Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149-53.
12. Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058-66.
13. Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72-9.
14. Amary MF, Bacsi K, Maggiani F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334-43.
15. Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29:4482-90.
16. Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510-22.
17. Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333:1453-5.
18. Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226:7-16.
19. Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, and FUBP1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3:709-22.
20. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021-6.
21. van den Bent MJ, Hartmann C, Preusser M, et al. Interlaboratory comparison of IDH mutation detection. J Neurooncol. 2013;112:173-8.
22. Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874-80.
23. Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743-50.
24. Capper D, Weissert S, Balss J, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20:245-54.
25. Camelo-Piragua S, Jansen M, Ganguly A, et al. A sensitive and specific diagnostic panel to distinguish diffuse astrocytoma from astrocytosis: chromosome 7 gain with mutant isocitrate dehydrogenase 1 and p53. J Neuropathol Exp Neurol. 2011;70:110-5.
26. Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118:401-5.
27. Bolduc JM, Dyer DH, Scott WG, et al. Mutagenesis and Laue structures of enzyme intermediates: isocitrate dehydrogenase. Science. 1995;268:1312-8.
28. Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225-34.
29. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739-44.
30. Chowdhury R, Yeoh KK, Tian YM, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463-9.
31. Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553-67.
32. Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17-30.
33. Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479-83.
34. Kaelin WG Jr, Thompson CB. Q&A: Cancer: clues from cell metabolism. Nature. 2010;465:562-4.
35. Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261-5.
36. Cairncross JG. Cognition in survivors of high-grade glioma. J Clin Oncol. 1998;16:3210-1.
37. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31:337-43.
38. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2013;31:344-50.
39. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Central nervous system cancers. Version 2.2012. Leptomeningeal metastases. 2012 [updated Dec 21, 2012; cited Feb 25, 2013]; Available from: www.nccn.org.
40. Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560-6.
41. Dubbink HJ, Taal W, van Marion R, et al. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73:1792-5.
42. Taal W, Dubbink HJ, Zonnenberg CB, et al. First-line temozolomide chemotherapy in progressive low-grade astrocytomas after radiotherapy: molecular characteristics in relation to response. Neuro Oncol. 2011;13:235-41.
43. van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985-90.
44. Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. 2008;109:835-41.
45. Vogelbaum MA, Berkey B, Peereboom D, et al. Phase II trial of preirradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas: RTOG BR0131. Neuro Oncol. 2009;11:167-75.
46. Blonski M, Pallud J, Goze C, et al. Neoadjuvant chemotherapy may optimize the extent of resection of World Health Organization grade II gliomas: a case series of 17 patients. J Neurooncol. 2013 Mar 12. [Epub ahead of print]
47. Keles GE, Chang EF, Lamborn KR, et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006;105:34-40.
48. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-8.
49. Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564-76; discussion 64-76.
50. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;
26:1338-45.
51. Cahill DP, Beiko J, Suki D, et al. IDH1 status and survival benefit from surgical resection of enhancing and nonenhancing tumor in malignant astrocytomas. J Clin Oncol. 2012;30:abstr 2019.