Gemcitabine Sensitizes Invasive Bladder Tumors to Radiation
Adding gemcitabine (Gemzar) to concurrent radiotherapy and cisplatinum in invasive bladder cancer could preserve the organ even when the tumor has invaded the muscle.
Adding gemcitabine (Gemzar) to concurrent radiotherapy and cisplatinum in invasive bladder cancer could preserve the organ even when the tumor has invaded the muscle, according to French researchers. In their pilot study, they sought to determine the maximum tolerated dose of gemcitabine to use with the gold standard of cisplatinum + radiotherapy.
Lead investigator David Azria, MD, PhD, noted that cystectomy is the gold standard for treatment of invasive bladder cancer, but because of a high morbidity rate, patients often opt for bladder preservation. With standard bladder preservation treatments, about 50% of patients have a functioning bladder at five years, said Dr. Azria, a professor in radiation oncology at the Centre Regional de Lutte contre le Cancer Val d'Aurelle-Paul Lamarque in Montpellier, France.
The patients in the study received a diagnosis of urothelial invasive bladder cancer but without hydronephrosis or diffuse carcinoma in situ. The cancer stage was T2-T4a with negative nodes and no metastasis.
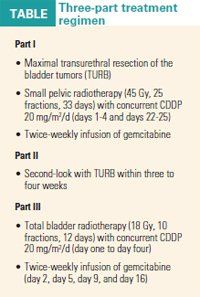
Concurrent radiotherapy and cisplatinum (CDDP) therapy was administered in a fixed dose to all the patients. Gemcitabine was dose escalated with twice-weekly infusions starting at 15 mg/m2. The treatment regimen was divided into three parts (see Table).
Of the 14 patients who were enrolled in the trial, nine were able to complete the regimen. Three patients did not receive the gemcitabine-radiation treatment, including the two with dose-limiting toxicities with gemcitabine.
Seven of the nine patients who completed the experimental regimen were cancer-free with intact bladders at a median follow up of 24 months.
Dr. Azria said no adverse events occurred at the 15 mg/m2, 20 mg/m2, and 25 mg/m2 doses. However, two serious adverse events-thrombocytopenia and severe performance status alteration-occurred with administration of the 30 mg/m2 dose of gemcitabine.
"The recommended dose of gemcitabine is 25 mg/m2 twice a week," Dr. Azria reported in the poster presentation (2010 Genitourinary Cancers Symposium abstract 305).
VANTAGE POINT Interesting data on chemosensitivity requires more support

The use of gemcitabine to sensitize tumors for chemoradiation appears to have beneficial early results, said Dr. Vogelzang, vice chair of the Southwest Oncology Group genitourinary oncology committee and medical director of the developmental therapeutics committee for US Oncology Research in Las Vegas.
"The work done by this French group is interesting," he said, but he cautioned that even though the dose of gemcitabine in this pilot study was small, the researchers did have drug-related adverse events. Dr. Vogelzang said that further studies using this treatment would be enlightening.