Gene Mutations May Affect Response to Chemotherapy
NEW ORLEANS-New findings reported at the 92nd Annual Meeting of the American Association for Cancer Research (AACR) support an emerging view that genetic mutations not only contribute to cancer etiology but even to individual variability in response to cancer treatment.
NEW ORLEANSNew findings reported at the 92nd Annual Meeting of the American Association for Cancer Research (AACR) support an emerging view that genetic mutations not only contribute to cancer etiology but even to individual variability in response to cancer treatment.
Small changes in a patient’s genetic makeup, for exampleeven the substitution of a single letter in the genetic codecan positively or negatively affect treatment response, according to several reports at the meeting. These findings point the way to a future of truly "personalized" cancer treatment, as pharmacogenetics will make it possible to tailor therapy to the genetic characteristics of a patient or the tumor.
"I am a zealot for this concept," said Jeffrey Trent, MD, of the NIH’s National Human Genome Research Institute. "We will begin to individualize patient treatment, based on genetic information. As to how genetics is impacting individual patients, I would say the future is now."
One study from Duke University Medical Center linked three previously identified genetic variations to length of survival in patients with metastatic breast cancer treated with several different agents. In one case, having the variation was beneficial, while having the other two variations proved detrimental.
The genes in this study mediate enzymes involved in the metabolism of many chemotherapy agents, said lead investigator William P. Petros, PharmD, assistant clinical professor of medicine at Duke and director of the Clinical Pharmacology Lab.
Dr. Petros and his colleagues stored white blood cell samples from 86 chemotherapy-naïve women with metastatic or inflammatory breast cancer before they began therapy with high-dose cyclophosphamide, cisplatin (Platinol), and car-mustine (BCNU).
DNA analysis showed that women with variations in the CYP3A4 and CYP3A5 genes were less able to activate cyclophosphamide, thus making this agent less efficacious. The median survival of these women was 12 to 18 months, compared with almost 3 years for women who did not have a variation in these two genes, Dr. Petros reported.
Furthermore, there were ethnic differences in the frequency of the variations. Few whites (12%) had the CYP3A4/CYP3A5 variations, compared with approximately 50% of blacks. Interestingly, the length of survival among blacks was about half that of white patients.
Survival was increased for women with no normal copies of a gene called GSTM1, compared with those who had one or two copies of the gene. In healthy white blood cells, this gene helps detoxify chemotherapy agents, thus protecting them from cytotoxicity. This gene may also detoxify chemotherapy agents in tumor cells, preventing the drug from working, Dr. Petros said.
The median survival for patients with both GSTM1 deletions was 3.8 years, compared to 1.8 years for patients with one or both GSTM1 copies. "In this case, having a mutation was a good thing," Dr. Petros said.
At a press conference, Dr. Petros said that genotype will dictate how drug dosages are figured for these agents, leading to less toxicity and better efficacy, and also may become factors to consider when evaluating outcomes of clinical trials.
P-glycoprotein Expression
Investigators from the University of Aberdeen, Scotland, also studied ethnic differences in chemotherapy response. They found a genetic mutation (C3435T) in all 10 ethnic groups they studied (1,280 subjects). However, the mutation was more common among Asians and whites than among persons of African descent.
The C3435T mutation results from the change of a single letter in the genetic code, reducing the expression of P-glycoprotein (PGP). PGP is known as the multidrug-resistance protein because it pumps chemotherapeutic agents out of tumor cells. The newly discovered mutation appears to be responsible for impaired PGP, making the cell less effective at eliminating the drugs.
Future studies will determine whether cancer patients with the PGP mutation respond better to chemotherapy. "If so, we will have identified a way to make therapy better for some patients. A patient’s PGP mutation status can help select the treatment regimen that is most likely to be effective," said lead investigator Howard L. McLeod, PharmD, currently at Washington University School of Medicine, St. Louis.
Gemcitabine Activity
In a third study, researchers found individual variation in the expression of two enzymes that regulate the activity of gemcitabine (Gemzar). In cells, gemcita-bine is phosphorylated to active metabolites by deoxycytidine kinase (dCK), the rate-limiting enzyme in the biotransformation of nucleoside analogs, while active metabolites of gemcitabine are inactivated through dephosphorylation by 5´-nucleotidase (5´-NT) (see Figure). Because these two enzymes act as opposing on-off switches for gemcita-bine, their ratios may influence drug cytotoxicity and thus help explain differences in patient response to the drug.
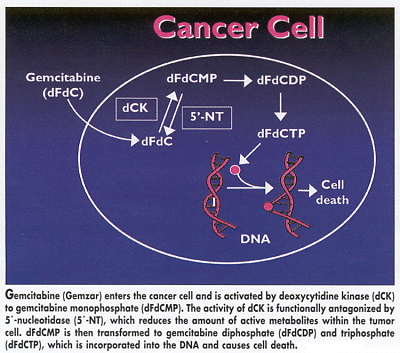
Italian researchers found that ratios of these two enzymes varied considerably in tumor specimens of non-small-cell lung cancer (NSCLC). The study suggests that researchers may someday be able to optimize treatment for NSCLC by evaluating the pharmacogenetics of drug-metabolizing systems before chemotherapy is begun, said Romano Danesi, MD, PhD, University of Pisa.