Good Safety Profile Reported for Biweekly Schedule of Capecitabine and Irinotecan
MADRID, SPAIN-Capecitabine(Xeloda) plus irinotecan (Camptosar),in a biweekly schedule as firstlinetreatment of locally advanced ormetastatic colorectal cancer, is an activeschedule with a manageable tox
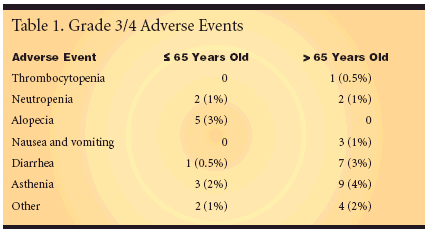
MADRID, SPAIN-Capecitabine(Xeloda) plus irinotecan (Camptosar),in a biweekly schedule as firstlinetreatment of locally advanced ormetastatic colorectal cancer, is an activeschedule with a manageable toxicityprofile, even in patients olderthan 65 years. This conclusion andsupporting results from a phase II trialwere reported by Pilar Garcia-Alfonso,MD, of Hospital Gregorio Maranonin Madrid (abstract 3540).Dr. Garcia-Alfonso said that thebiweekly schedule of capecitabine/irinotecan (XELIRI) was less toxic thanthe standard 3-weekly XELIRI regimen.All 45 patients enrolled in thetrial were evaluable for safety. Grade3/4 toxicities were uncommon (Table1). The most common grade 3/4 toxicityamong patients ≤ 65 years wasalopecia, occurring in 5 patients (3%)younger than age 65 but in no patientsolder than 65. The most common adverseevents among these older patientswere asthenia (nine patients[4%]) and diarrhea (seven patients[3%]).50% Response RateThe overall response rate amongthe 38 patients evaluable for efficacywas 50% (95% confidence interval[CI]: 33%-67%). This total included 3patients with complete responses and16 patients with partial responses. Thetumor-growth control rate (responserate plus stable disease rate) was 87%(CI 95%: 72%-96%). Dr. Garcia-Alfonsosaid that a total of 50 patientswill be enrolled to evaluate time toprogression.
The 2-week treatment cycle consistedof 175 mg/m2 of irinotecan administeredon day 1 as a 90-minuteinfusion and 1,000 mg/m2 of oralcapecitabine twice daily on days 2 to 8.For patients older than age 65, theirinotecan dose was reduced to 140mg/m2 and the capecitabine dose, to750 mg/m2 twice daily. The medianrelative dose intensity was 94% forcapecitabine and nearly 100% foririnotecan.The median age of the patients was67 years (range, 42-80 years), with 25patients (56%) older than age 65. EasternCooperative Oncology Group(ECOG) performance status was 0 to 1in 93% of patients. Primary tumorsites were the colon (22 patients[51%]), rectum (21 patients [47%]),or both (1 patient [2%]). The mediannumber of metastatic lesions was two,and the most common sites were theliver (56%) and the lungs (23%).Previous treatment, such as surgery(73%), adjuvant chemotherapy(44%), and radiotherapy (20%), wasallowed if completed ≥ 6 months beforeenrollment.