Graft-Versus-Host Disease: A Complex Long-Term Side Effect of Hematopoietic Stem Cell
Consider the following case study, which illustrates the complex physical and psychosocial care required for the patient developing graft-versus-host disease (GVHD) following an allogeneic hematopoietic stem cell transplantation (HSCT): Mr. SR is a 38-year-old male with a diagnosis of anaplastic large cell non-Hodgkin’s lymphoma (NHL).
ABSTRACT: Hematopoietic stem cell transplantation (HSCT)/ bone marrow transplantation (BMT) has become a central treatment modality in the management of various hematologic malignancies, but it is not without treatment sequelae. The major complication of HSCT/BMT is acute or chronic graft-versus-host disease (GVHD). GVHD is an immunologically mediated disease that contributes substantially to transplant-related morbidity and mortality. The overall incidence of GVHD remains between 30% and 60% and carries approximately a 50% mortality rate. Acute and chronic GVHD are complex clinical phenomena. This paper focuses on our current clinical understanding of GVHD as a multiphase process intricately linked to the immune response between the donor (graft) and recipient (host). Based on this complex pathophysiology, clinical nursing care is targeted at the various body systems affected by either acute or chronic GVHD. The article concludes with advances and clinical trials underway that have the potential to reduce the symptomatology of GVHD.
Consider the following case study, which illustrates the complex physical and psychosocial care required for the patient developing graft-versus-host disease (GVHD) following an allogeneic hematopoietic stem cell transplantation (HSCT): Mr. SR is a 38-year-old male with a diagnosis of anaplastic large cell non-Hodgkin’s lymphoma (NHL).
Originally diagnosed in March 2005, Mr. SR’s lymphoma recurred after treatment with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) followed by CHOP-R (with rituximab [Rituxan]) chemotherapy. At this point he was evaluated for an HSCT and was typed for a human leukocyte antigen (HLA) allogeneic donor match. Initially Mr. SR underwent an autologous transplant. However, after only 20 days his lymphoma was evident, and planning was begun for an allogeneic transplant. While waiting for an allogeneic donor, Mr. SR received vinblastine and achieved a complete response with this chemotherapy, but his disease recurred shortly thereafter. Finally, it was determined that his sister was an HLA match and Mr. SR underwent an allogeneic transplant; on day 28 his NHL recurred, requiring additional chemotherapy (vinblastine).
Mr. SR was given immunosuppressive agents to prevent GVHD. Initially he was given cyclosporine and CellCept (mycophenolate mofetil) as GVHD prophylaxis. As his CellCept was tapered, he developed a GVHD flare and was admitted for treatment with IV (intravenous) cyclosporine and solumedrol. Eventually Mr. SR was put on oral prednisone. As his prednisone was tapered, Mr. SR developed a flare of his liver GVHD, which was treated with Rituxan (rituximab). His CellCept was restarted along with a standing order for prednisone and cyclosporine.
Subsequently, Mr. SR developed GVHD. Initially his GVHD was mild and questionable. It took clinicians 1 month to diagnose GVHD. During the following year, the degree and intensity of his GVHD varied and it was difficult to manage. He recovered from one flare only to develop another. Mr. SR developed sicca syndrome; oropharyngeal GVHD; and hyper- and hypopigmented skin that was described as having scattered reddish, punctate papules on the chest and extremities and patchy, scaly, macular erythemia over the back.
TABLE 1

Mr. SR's Medication Regimen for cGVHD
The condition of Mr. SR’s skin made him feel “like a freak,” and when he asked his family for help with washing his back he was told that they were “grossed out” by its appearance. At their worst, Mr. SR’s hands were blistered and edematous. Mr. SR is being treated with a post-transplant regimen that includes cyclosporine, mycophenolate mofetil (CellCept), cyclosporine ophthalmic emulsion (Restasis), and prednisone. (See Table 1 for more details about these medications.)[1–3] Additionally Mr. SR received rituximab (Rituxan) therapy for sicca syndrome and recently started photopheresis therapy to help control his skin GVHD.
Psychosocially, the diagnosis and subsequent treatments including the HSCT and GVHD strained his marriage. The steroid requirement necessary to control his GVHD contributes to his emotional lability. He is short-tempered with his family. His wife has asked him for a divorce, stating that she “could not deal with his illness anymore,” although they are presently working with social services for counseling and possible reconciliation. Currently SR lives with his uncle, and financial concerns continue to be a problem.
Introduction
GVHD is an immunologically mediated disease that contributes substantially to transplant-related morbidity and mortality. The overall incidence of GVHD remains between 30% and 60% and carries a mortality rate of approximately 50%.[1,2,4] About 35% to 50% of HSCT recipients will develop acute GVHD. The exact risk is dependent on the stem cell source, age of the patient, conditioning, and GVHD prophylaxis used. Given the number of transplants performed, we can expect about 5,500 patients per year to develop acute GVHD, while the incidence of chronic GVHD is even higher.[5] Chronic GVHD occurs in at least 30% to 50% of recipients of transplants from human leukocyte antigen (HLA) matched siblings, and in at least 60% to 70% of recipients from unrelated donors.[6–10]
Despite all of the scientific gains in HSCT, the complication of GVHD remains a complex post-transplant problem. In allogeneic HSCT there are mismatches in major histocompatibility complex (MHC) antigens resulting in GVHD when immunologically competent T cells from the donor graft attack the host. This article presents an overview of GVHD, including pathophysiology, clinical manifestations, and medical and nursing care for the patient with this potentially life-threatening complication of HSCT.
Overview of Graft-Versus-Host Disease
GVHD can be either an acute (aGVHD) or chronic (cGVHD) condition, with clinical damage to three target organs: skin, gastrointestinal (GI) tract, and liver in aGVHD; and to additional organ systems in cGVHD. Three criteria specified for the development of GVHD are as follows: 1) The graft must contain immunologically competent cells; 2) The host must appear foreign to the graft and be capable of stimulating the donor cells; and 3) The host immune system must be incapable of mounting an effective immunologic reaction against the graft for a period of time long enough for the graft to become sensitized and mount an immunologic assault on the host. These antigenic differences stimulate the donor lymphocytes to attack epithelial cells and mucous membranes in the skin, intestinal tract, and the biliary ducts, and one or more organs may be involved. A clinical diagnosis is supported with tissue biopsies to help differentiate GVHD from other diagnoses.[11,12]
The current model of GVHD presents a multiphase process. In phase one, the conditioning regimen results in tissue damage with release of cytokines. The transplant conditioning regimen intentionally causes host tissue injury and ablates the host immune response so that the HSCT will engraft. The high-dose conditioning regimens are toxic to many organ systems, including the skin, liver, and GI tract.[13] Preparative regimens, using chemotherapy such as cyclophosphamide and radiation therapy, directly damage recipient tissue, and this damage begins an inflammatory cytokine cascade. The production and release of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-12 (IL-12) enhance antigen presentation and adhesion molecule expression. These cytokines activate host antigen-presenting cells (APCs). The antigen presentation is caused by the upregulation of the MHC and minor histocompatibility antigens (mHA). The adhesion molecule expression causes white blood cells to be attracted to and retained in the damaged area and donor T lymphocytes to attack recipient tissues.[12,14,15]
In phase two, donor T cells recognize alloantigens on host APCs. These activated T cells then proliferate, differentiate into effector cells, and secrete cytokines, particularly interferon-gamma (IFN-γ). These cytokines then support and drive the proliferation of donor T cells responding to the host antigens. The greater the immunologic disparity between the donor and recipient, the greater the T-cell response. The inflammatory cytokine cascade continues with subsets of T lymphocytes; T helper-1 lymphocytes also produce proinflammatory cytokines, including IL-12, interleukin-2 (IL-2), and interferon-γ. Additionally cytotoxic T lymphocytes and natural killer cells respond and stimulate monocytes to produce IL-1 and TNF-α, resulting in direct tissue damage of the skin and gut.[12,16]
In phase three, target cells undergo apoptosis mediated by cellular effectors (eg, donor cytotoxic T lymphocytes) and inflammatory cytokines such as TNF-α. TNF-α secretion is amplified by stimuli such as endotoxin that leaks across damaged gastrointestinal mucosa injured by the chemoradiotherapy in the first phase. T cells also directly attack host tissue. The ongoing tissue damage results in further cytokine production, thus perpetuating the cascade. TNF-α and IFN-γ cause further injury to gastrointestinal epithelium, causing more endotoxin leakage and establishing a positive inflammatory feedback loop. The resulting cytokine production is often referred to as a cytokine storm.[7,17]
FIGURE 1
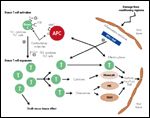
Three-phase model of graft-versus-host disease (GVHD)
In aGVHD the preparative regimen causes the release of inflammatory cytokines and increases the expression of MHC antigens within the host. The MHC antigens are involved in the steps leading to T-cell activation. They contain genes that encode tissue antigens used for tissue typing and these genes lie on the short arm of chromosome 6, the HLA. Even when HLA typing appears matched, patients still develop GVHD. This is thought to be secondary to minor histocompatibility antigen differences which are expressed on the cell surface as degraded peptides bound to specific HLA molecules.[18–21] Figure 1 is an illustration of the complex mechanisms that are operational in aGVHD.[22]
Chronic GVHD is one of the most common problems and severe complications affecting patients surviving >100 days after allogeneic HSCT.[23,24] It is considered a syndrome of immune dysfunction that results in immunodeficiency and autoimmunity.[25–27] The pathophysiology of cGVHD remains largely unknown. Theories suggest that cGVHD may be the result of end-stage alloreactivity from T cells,[28] or caused by poor or dysfunctional immunologic recovery, [22] or the result of autoreactive clones normally deleted in the thymus, although if the thymus is damaged the result is formation of autoantibodies similar to those seen in autoimmune disease.[10]
The usual time frames for occurrence of aGVHD (time of engraftment to 100 days post-transplant) and cGVHD (after 100 days) have become complicated with the use of donor lymphocyte infusion (DLI). Acute GVHD may occur initially post DLI, requiring the need to keep track of the “day” post transplant and the “day” post DLI.
Clinical Management
TABLE 2
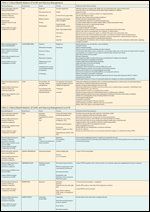
Clinical Manifestations of GVHD and Nursing Management
The clinical management of patients with GVHD is challenging. Once the diagnosis of GVHD is confirmed and staged, frequent assessment is necessary to determine the response to therapy and to assist with comfort and healing.[29] Patients should be monitored for their GVHD in a standardized fashion using formal GVHD documentation tools.[8,30] It is important that patients be monitored consistently to allow early and effective intervention for this major complication of HSCT. Nursing care of the patient is focused on detection of early signs and symptoms, support of patient comfort, and implementation of the medical plan of care, including fluid and electrolyte replacement, antidiarrheal therapy, antibiotic (particularly antifungal) therapy, immunosuppressive therapy, and nutritional support. Topical corticosteroid creams may be applied to the skin in patients with minimal skin involvement. In addition, patients with gut involvement may require narcotic analgesia to control pain. Table 2 provides an extensive nursing care plan for the patient with acute and chronic GVHD.
The mainstay of medical management uses medications for prophylaxis and treatment of GVHD (see Table 3).[31] If aGVHD develops after transplantation, usually methylprednisolone or prednisone in combination with cyclosporine is administered. Satisfactory responses to this steroid treatment are observed in 50% to 75% of patients. New drugs and strategies that can supplement standard treatment are now available or are in clinical trials, including monoclonal antibodies (eg, anti-CD3, anti-CD5, and IL-2 antibodies), mycophenolate mofetil alemtuzumab (Campath), antithymocyte globulin, tacrolimus, and sirolimus.[9,32–35]
TABLE 3
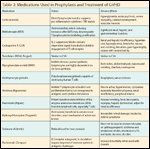
Medications Used in Prophylaxis and Treatment of GVHD
The primary therapy for cGVHD is administration of steroids, such as prednisone, azothioprine, and cyclosporine, that suppress the patient’s immune system. Clinical trials investigating treatments of steroid-refractory chronic GVHD include use of tacrolimus, mycophenolate mofetil, thalidomide, daclizumab, extracorporeal photopheresis, infliximab (Remicade), and clofazimine (Lamprene), with reported success rates of between 25% and 50%.[36–41] Antibiotics such as trimethoprim/sulfamethoxazole (Bactrim, Septra) or penicillin or both are usually taken to reduce the risk of infection.
In addition, patients may be required to wear face masks in the presence of other people; stay out of crowds; and avoid fresh plants, fruits, and vegetables. Patients with cGVHD are usually advised to avoid vaccinations with live viruses such as German measles, tetanus, and polio, until the GVHD problem is completely resolved and the use of immunosuppressive drugs ends.
A National Institutes of Health (NIH) Consensus Conference established guidelines for ancillary therapy and supportive care in cGVHD. The conference report outlines recommended treatments for symptoms and gives recommendations for patient education, preventive measures, and appropriate follow-up. Also developed were standard criteria for the diagnosis of cGVHD and a proposed new clinical scoring system that describes the extent and severity of cGVHD for each organ or site. In 2006 the National Institutes of Health Consensus Conference established guidelines for ancillary therapy and supportive care in cGVHD.[5,30,42]
Infection
The primary cause of death in patients with GVHD is infection, and thus there must be a high index of suspicion for infection in this patient population. All patients should receive antimicrobial prophylaxis. Patients with aGVHD should receive prophylaxis for Pneumocystis carinii with agents such as trimethoprim/sulfamethoxazole, dapsone, atovaquone (Mepron), or pentamidine. Patients with cGVHD should also receive prophylaxis, such as penicillin, against encapsulated organisms including pneumococcus (Streptococcus pneumoniae). Patients are maintained for a lifetime on antimicrobials for prophylaxis against pneumococcus.
Additionally, patients should receive antibiotic prophylaxis for dental and all other invasive procedures, according to the endocarditis prophylaxis recommendations of the American Heart Association. Topical antifungal prophylaxis with clotrimazole (Mycelex) troches or nystatin swish and swallow should be used in all patients receiving topical steroids for oral GVHD. Reactivation of virus is a problem in patients with acute and chronic GVHD,[43] and patients should be monitored for cytomegalovirus. Many patients have outbreaks of oral and genital herpes and should receive prophylaxis if they are receiving high doses of immunosuppressive agents. Educate patients about increased risk of infection, even if they are not undergoing immunosuppression.
PhotophEresis Treatment
Skin care for the patient with GVHD is based upon early detection of signs and symptoms. Many patients exhibit GVHD with red rash, itching, and darkening of the skin. The patient can help himself by resisting the urge to scratch, using moisturizing lotion and sunscreen with an SPF of 30 or greater daily, avoiding hot showers and prolonged sun exposure, and wearing long sleeves and pants while he is outdoors.
Steps that are commonly used in the treatment are fluid and electrolyte replacement, antifungal therapy, immunosuppressive therapy, and nutritional support. Topical corticosteroids may also be applied to the skin in patients with minimal involvement. Chronic skin GVHD is treated with steroids such as prednisone and cyclosporine, which suppress the patient’s immune system. Antibiotics such as trimethoprim/sulfamethoxazole or penicillin are usually taken orally to reduce risk while cGVHD is being treated with IV antibiotics.
PUVA is a combination of psoralen (P) and long-wave ultraviolet radiation (UVA) that is used to treat cGVHD. Psoralen is a drug taken by mouth that makes the skin disease more responsive to ultraviolet light. PUVA is likely to be a good option in steroid-refractory cGVHD but seems to also have some degree of activity in aGVHD as well. The T cells in the dermis are sensitized to UVA light by the psoralen and are then inactivated by cutaneous UVA irradiation. During PUVA treatments, cutaneous T cells lose the ability to migrate and to proliferate upon antigen stimulation. The mechanism for this is not clear.
Therapy typically needs to be repeated weekly or biweekly until resolution or no evidence of improvement. Response is sometimes delayed, and a minimum of 4–6 doses is recommended before evaluation can be made. The addition of acitretin (Soriatane) to the treatment regimen has helped some patients, while others have benefited from the use of clofazimine or hydroxychloroquine (Plaquenil). A less toxic alternative is extracorporeal photopheresis (ECP): White blood cells are removed from the patient, incubated with psoralen, exposed to ultraviolet light, and then reinfused into the patient. In preliminary reports, five of eight patients showed improvement after ECP.[37,44,45] Physical and/or occupational therapy may help cGVHD patients with scleroderma to maintain their flexibility and range of motion. Myofascial release and massage has helped some patients with facial scleroderma. PUVA treatments for the mouth are also a treatment possibility.
Future Trends in Treatment of GVHD
Novel therapies include trying to prevent GVHD at the cellular level. Acute GVHD limits the application of this curative but toxic therapy. Studies of inflammatory pathways involved in GVHD show that the gastrointestinal (GI) tract plays a major role in the amplification of systemic disease. Damage to the GI tract increases inflammatory stimuli such as endotoxin, promoting further inflammation and additional GI tract damage. Experimental approaches to the prevention of aGVHD include reducing the damage to the GI tract by fortification of the GI mucosal barrier through novel “cytokine shields” such as IL-11 or keratinocyte growth factor. Such strategies have reduced aGVHD while preserving a graft-versus-leukemia effect in animal models, and they are being formally tested in carefully designed clinical trials.[46]
Manipulation of T cells and techniques that selectively deplete only alloactivated donor T cells from donor grafts look promising.[47] Studies of DNA-based tissue typing increase the accuracy of matched donors, consequently decreasing GVHD, and use of less mature cells, such as those from cord blood, is associated with a decrease in GVHD.[3] The development of new drugs, combined with our understanding of GVHD, may decrease the severity as well as the morbidity and mortality of this major complication in HSCT, leading patients to long-term survival and better quality of life.
Care of the HSCT patient with cGVHD is complex, with many physical and psychosocial issues, as evidenced in the case of Mr. SR. Chronic GVHD is the major cause of nonrelapse mortality in patients undergoing HSCT. Studies show that standard treatments, such as steroids, work only to a certain point.[48,49] New and improved therapies are sought to manage cGVHD and to limit exposure to steroids. ECP is a treatment showing promise in preliminary studies.[50–52] However, there are drawbacks to ECP, such as limited availability, cost, frequency and duration of treatment, and long-term side effects, all of which impact the patient’s and the family’s quality of life.
To date, efforts to prevent the development of GVHD have been unsuccessful. New trends in transplant, such as increased age of patients undergoing HSCT, increase in the number of mismatched antigens, and use of nonmyeloablative therapies will continue to make GVHD a progressively common problem.
References:
References
1. Ezzone S, Schmit-Pokorny K: Blood and Marrow Stem Cell Transplantation Principles, Practice, and Nursing Insights, 3rd ed. Sudbury, MA, Jones and Bartlett Publishers, 2007.
2. Mattson MR: Graft-versus-host disease: Review and nursing implications. Clin J Oncol Nurs 11(3):325â328, 2007.
3. Thomas ED, Blume KG, Forman SJ: Hematopoietic Cell Transplantation, 2nd ed. Malden, MA, Blackwell Science, Inc., 1999.
4. Antin J: Long-term care after hematopoietic-cell transplantation in adults. N Engl J Med 347:36â42, 2002.
5. Jacobsohn DA, Vogelsang GB: Acute graft-versus-host disease. Orphanet J Rare Dis 4(2):35, 2007.
6. Couriel D, Caldera H, Champlin R, et al: Acute graft-versus-host disease: Pathophysiology, clinical manifestations, and management. Cancer 101:1936â1946, 2004.
7. Ferrara JL, Cooke KR, Teshima T: The pathophysiology of acute graft-versus-host disease. Int J Hematol 78, 181â184.
8. Lee SJ, Vogelsang G, Flowers ME: Chronic graft-versus-host disease. Biol Blood Marrow Transplantation 9:215â233, 2003.
9. Pavletic SZ, Martin P, Lee SJ, et al: Measuring therapeutic response in chronic graft versus host disease. Biol Blood Marrow Transplantation 12(3): 252â266, 2006.
10. Sullivan KM: Graft-versus-host-disease. In Thomas ED, Blume KG, Forman SJ (eds): Hematopoietic Cell Transplantation, 2nd ed, pp 515â536. Malden, MA, Blackwell Science, Inc., 1999.
11. Dean DM, Bishop MR: Graft-versus-host disease: Emerging concepts in prevention and therapy. Curr Hematol Rep 2(4):287â294, 2003.
12. Mitchell SA: Graft-versus-host disease. In Ezzone S (ed): Hematopoietic Stem Cell Transplantation: A Manual for Nursing Practice, pp 85â131. Pittsburgh, PA, Oncology Nursing Society, 2004.
13. Hill GR, Ferrara JL: The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 95:2754â2759, 2000.
14. Deeg HJ: Cytokines in graft-versus-host disease and the graft-versus-leukemia reaction. Int J Hematol 74(1):26â32, 2001.
15. Sun Y, Tawara I, Toubai T, et al: Pathophysiology of acute graft-versus-host disease: Recent advances. Transl Res 150(4), 197â214, 2007.
16. Weisdorf D: GVHD: The nuts and bolts. Hematology Am Soc Hematol Educ Program 2007(1):62â 67, 2007.
17. Couriel DR, Saliba RM, Giralt S, et al: Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant 10(3):178â185, 2004.
18. Kim H: Mini-allogeneic stem cell transplantation. Cancer Pract 10(3):170â172, 2002.
19. Miura Y, Thoburn CJ, Bright EC, et al: Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood 104(7):2187â2193, 2004.
20. Morris ES, Hill G: Advances in the understanding of acute graft-versus-host disease. Br J Haematol 137(1):3â19, 2007
21. Petersdorf EW, Hansen JA, Martin PJ, et al: Major-histocompatibility-complex class I alleles and antigens in hematopoietic-cell transplantation. N Engl J Med 345(25):1794â1800, 2001.
22. Vogelsang GB, Lee L, Bensen-Kennedy DM: Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med 54:29â52, 2003.
23. Bhushan V, Collins RH: Chronic graft-vs-host disease. JAMA 290(19):2599â2603, 2003.
24. Przepiorka D, Weisdorf D, Martin P: 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 15(6):825â828, 1995.
25. Lee SJ: New approaches for preventing and treating chronic graft-versus-host disease. Blood 105(11):4200â4206, 2005.
26. Pérez-Simón JA, Sánchez-Abarca I, DÃez-Campelo M, et al: Chronic graft-versus-host disease: Pathogenesis and clinical management. Drugs 66(8):1041â1057, 2006.
27. Williams, L: Comprehensive review of hematopoiesis and immunology: Implications for hematopoietic stem cell recipients. In Ezzone S (ed): Hematopoietic Stem Cell Transplantation: A Manual for Nursing Practice, pp 1â12. Pittsburgh, PA, Oncology Nursing Society, 2004.
28. Kataoka Y, Iwasaki T, Kuroiwa T, et al: The role of donor T cells for target organ injuries in acute and chronic graft-versus-host disease. Immunol 103(3):310â318, 2001.
29. Jacobsohn DA, Montross S, Anders V, et al: Clinical importance of confirming or excluding the diagnosis of chronic graft-versus-host disease. Bone Marrow Transplant 28(11):1047â1051, 2001.
30. Pavletic SZ, Vogelzang GB: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant 11(12):943â944, 2005.
31. Anders V, Barton-Burke M: Graft-versus-host disease: Complex sequelae of stem cell transplantation. In Ezzone S, Schmit-Pokorny K (eds): Blood and Marrow Stem Cell Transplantation Principles, Practice, and Nursing Insights, 3rd ed, pp 147â181. Sudbury, MA, Jones and Bartlett Publishers, 2007.
32. Antin JH: Clinical practice. Long-term care after hematopoietic-cell transplantation in adults. N Engl J Med 347(1):36â42, 2002.
33. Bacigalupo A, Palandri F: Management of acute graft-versus-host disease (GvHD). Hematol J 5(3):189â196, 2004.
34. Bolwell B, Sobecks R, Pohlman B, et al: A prospective randomized trial comparing cyclosporine and short-course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant 34(7):621â625, 2004.
35. Przepiorka D, Kernan NA, Ippoliti C, et al: Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood 95(1):83-89, 2000.
36. Bolanos-Meade J, Jacobsohn DA, Margolis J, et al: Pentostatin in steroid refractory acute graft-versus-host disease.J Clin Oncol 23(12):2661â2668, 2005.
37. Breuckmann F, Gambichler T, Altmeyer P, et al: UVA/UVA1 phototherapy and PUVA photochemotherapy in connective tissue disease and related disorders: A research based review. BMC Dermatol 4(11):1471â1594, 2004.
38. Kobbe G, Schneider P, Rohr U, et al: Treatment of severe steroid refractory acute graft-versus-host disease with infliximab, a chimeric human/mouse anti-TNF alpha antibody. Bone Marrow Transplant 28(1):47â49, 2001.
39. Koc S, Leisenring W, Flowers ME, et al: Therapy for chronic graft-versus host disease: A randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 100(1):48â51, 2002.
40. Vogelsang GB, Arai S: Mycophenolate mofetil for the prevention and treatment of graft-versus-host disease following stem cell transplantation: Preliminary findings. Bone Marrow Transplant 27(12):1255-1262, 2001.
41. Vogelsang GB, Farmer ER, Hess AD, et al: Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med 326(16):1055â1058, 1992.
42. Couriel D, Carpenter P, Cutler C, et al: Ancillary therapy and supportive care of chronic graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant 12(4):375â396, 2006.
43. Lin TS, Zahrieh D, Weller E, et al: Risk factors for cytomegalovirus reactivation after cd6+ t-cell depleted allogeneic bone marrow transplantation. Transplantation 74(1):49â54, 2002.
44. Alcindor T, Gorgun G, Miller KB, et al: Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood 98(5):1622â1625, 2001.
45. Woltz P, Castro K, Park BJ: Care for patients undergoing extracorporeal photopheresis to treat chronic graft-versus-host disease: Review of the evidence. Clin J Oncol Nurs 10(6):795â802, 2006.
46. Wilkes G, Barton-Burke M: Oncology Nursing Drug Handbook. Sudbury, MA, Jones and Bartlett Publishers, 2008.
47. Pavletic SZ, Carter SL, Kernan NA, et al: Influence of T cell depletion on chronic graft-versus-host disease: Results of a multi-center randomized trial in unrelated marrow donor transplantation. Blood 106:3308â3313, 2005.
48. Zhang Y, Hexner E, Frank Dui, et al: CD4+ T cells generated de novo from donor hematopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol 179(5):3305â3314, 2007.
49. Ho V, Zahrieh D, Hochberg E, et al: Safety and efficacy of denileukin diftitox in patients with steroid refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood 104(4):1224â1226, 2004.
50. Furlong T, Leisenring W, Storb R, et al: Psoralen and ultraviolet A irradiation (PUVA) as therapy for steroid-resistant cutaneous acute graft-versus-host disease. Biol Blood Marrow Transplant 8(4):206â212, 2002.
51. Greinix H, Volv-Platzer B, Rabitsch W, et al: Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood 92(9):3098â3104, 1998.
52. Seaton ED, Szydlo RM, Kanfer E, et al: Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood 102(4):1217â1223, 2003.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.