Hand-Foot Skin Reaction Secondary to Sunitinib in a Patient With Metastatic Clear Cell Renal Cell Carcinoma
Another installment of Clinical Quandaries is presented by Karen Férez-Blando, MD, and colleagues of a 45-year-old man with a diagnosis of intermediate-risk stage IV clear cell renal carcinoma who develops grade 2 hand-foot skin reaction on sunitinib therapy.
Oncology (Williston Park). 2021;35(5):272-276.
DOI: 10.46883/ONC.2021.3505.072
Figure 1. Hyperkeratotic Plaques. These plaques, on the undersurface of the great toes and heels of both feet, occur predominantly at sites of pressure and friction.
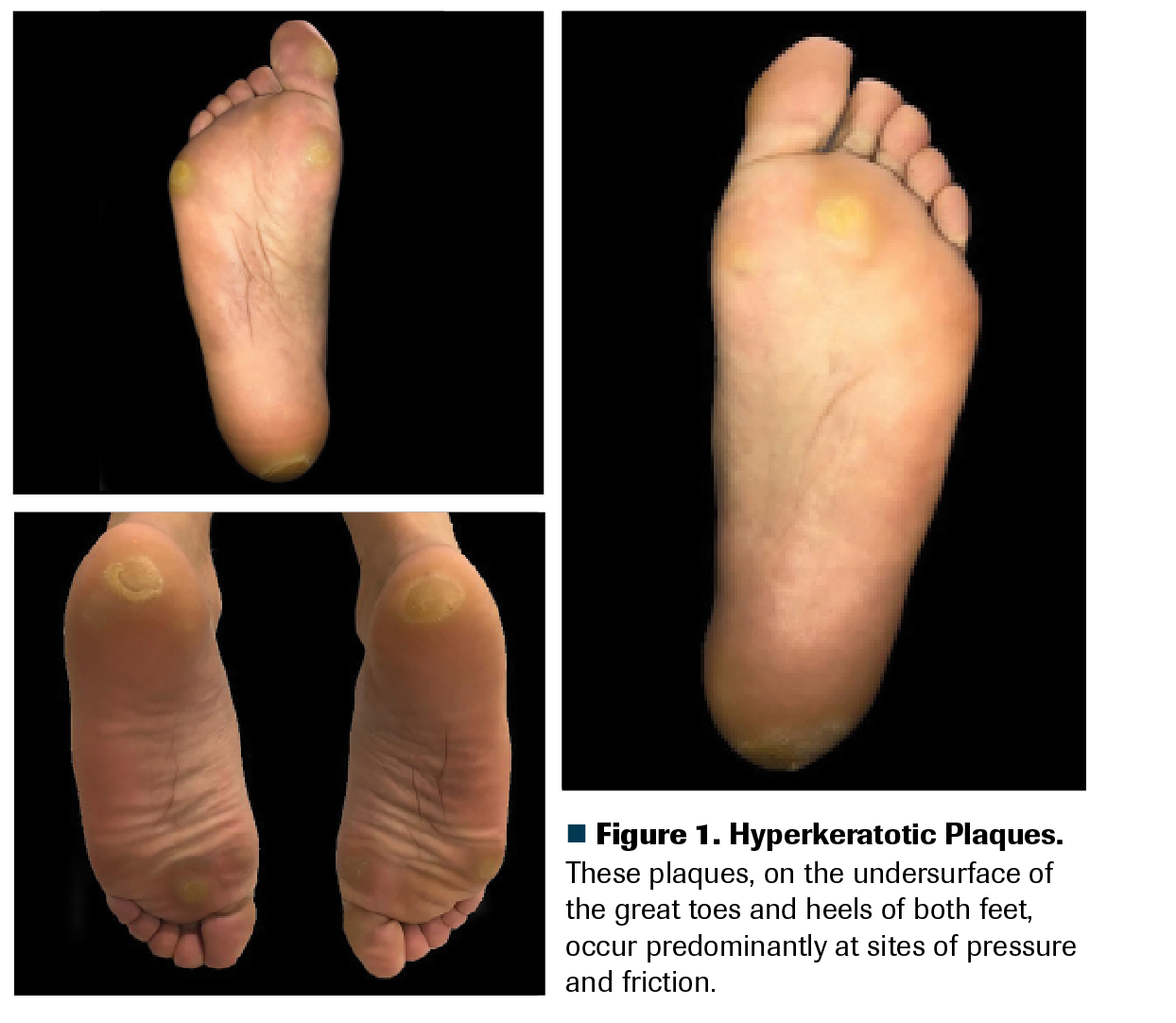
A man, age 45 years, was diagnosed with intermediate-risk stage IV clear cell renal carcinoma (lung and lymph node metastases). He was prescribed first-line systemic treatment with sunitinib (Sutent) 50 mg per day (each cycle: 4 weeks on, 2 weeks off). Upon day 22 of his second sunitinib cycle, he came to the oncology clinic complaining of difficulty walking due to bilateral sole pain. He described initial tingling sensations, which then became burning and painful, with symmetrical erythema and edema of the soles, without blisters. These turned into painful plaques with yellowish discoloration and hyperkeratosis on pressure-bearing areas. He denied fever or other symptoms. The pain limited his instrumental activities of daily living, but not his self-care activities of daily living. Total body skin examination disclosed hyperkeratotic plaques on the undersurface of the great toes and heels of both feet, predominantly at sites of pressure (Figure 1); no blisters, crusts, ulcers, or fissures were found. No relevant findings were found upon physical examination of his hands, mucosae, and scalp. A diagnosis of grade 2 hand-foot skin reaction (HFSR) was made.
Besides oral analgesics, which of the following is the best treatment for this patient?
Férez-Blando is a dermatologist in the Hematology/Oncology Department at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.

Castro-Alonso is a clinical researcher at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.

Domínguez-Cherit is an associate professor and head of the Department of Dermatology and National Researcher at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.

Bourlon is an associate professor and head of the Urologic Oncology Clinic, National Researcher. Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Mexico City, Mexico. She is also a member of the American Society of Clinical Oncology’s IDEA Working Group.

Discussion
Hand-foot syndrome (HFS) is a well-described cutaneous adverse event (AE) of certain chemotherapeutic agents, like capecitabine, docetaxel, 5-fluorouracil, and pegylated liposomal doxorubicin. It was first described in 1974 by Zuelke; alternative names for this AE include acral erythema, palmar-plantar erythrodysesthesia, and Burgdorf reaction.1 Clinical dermatological manifestations may include erythema, edema, dryness, blisters, fissures, ulcers, and/or desquamation. Varying degrees of paresthesia, dysesthesia, and pain can accompany such changes.2 Although palms and soles are the most commonly affected areas, changes in the dorsal surfaces and nails can occur.3
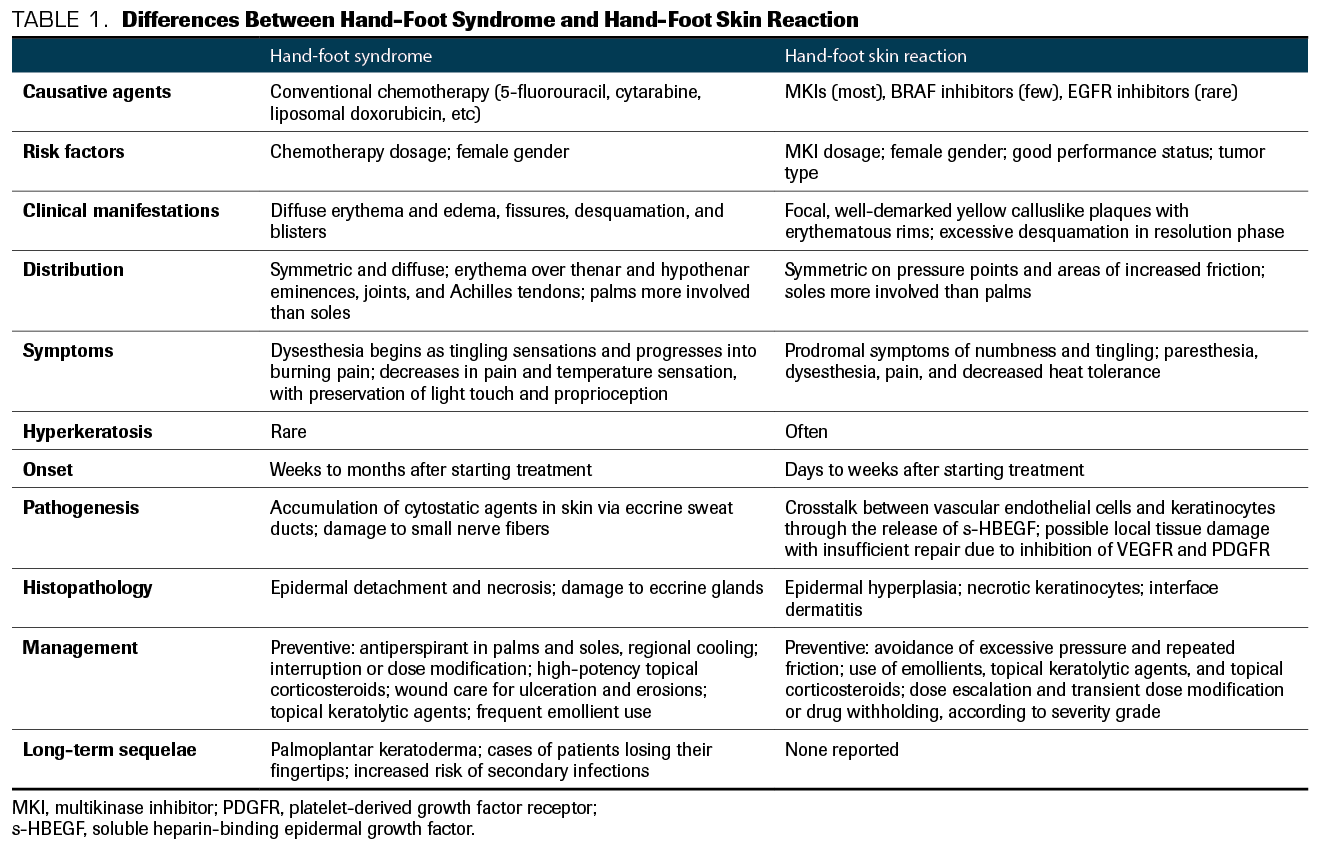
With the advent of targeted therapies such as multikinase inhibitors (MKIs), a novel profile of cutaneous AEs has emerged. Since the MKI-related skin changes affecting the palms and soles differ from the ones caused by chemotherapy, the term HFSR is used when referring to the MKI-induced syndrome.4 The differences between HFS and HFSR are summarized in Table 1. HSFR has been associated with various MKIs, including regorafenib (Stivarga), cabozantinib (Cabometyx), sorafenib (Nexavar), sunitinib, axitinib (Inlyta), and pazopanib (Votrient).5 Although it isn’t life-threatening, HFSR can lead to cessation of therapy, dose reduction, and impaired quality of life.6
Table 1. Differences Between Hand-Foot Syndrome and Hand-Foot Skin Reaction
HFSR affects 31.2% to 79.4% of patients using MKIs.7-9 The frequency of all-grade HFSR differs greatly among the MKIs—for instance, the frequency with regorafenib is 46% to 84%; with cabozantinib, 35%; sorafenib, 10% to 62%; and sunitinib, 10% to 28%. The corresponding frequencies for grade 3 or greater HFSR are 20.4%, 9.5%, 2% to 36%, and 4% to 12%, respectively. Notably, the incidence of HFSR from a specific MKI can vary among tumor types, as different doses might be indicated for them.1,10,11
HFSR has several risk factors. In a meta-analysis of randomized controlled trials involving 24,956 patients, the main risk factors for HFSR were:
(A) Tumor type. The highest risk was with thyroid cancer, with a relative risk (RR) of 12.62; the RR for renal cell carcinoma was 8.10.
(B) MKI used. Cabozantinib represented the highest RR with 12.10; the RR with sunitinib was 2.97.
(C) Dosing. Severity and frequency of HFSR positively correlated with the dosage of MKI.12
(D) Additional risk factors. These include female sex, good performance status, preexisting calluses, poorly fitting shoes, and repeated friction to the hands and feet.5
The pathogenesis of HFSR is still unclear. Proposed mechanisms include direct cytotoxic effect of the MKIs to the epidermis, eccrine secretion of MKI, and MKI-induced deficient capillary maintenance in high-friction areas.9,13
A crosstalk between vascular endothelial cells and keratinocytes has also been suggested as an etiological mechanism. In this model, MKI triggers the release of soluble heparin-binding epidermal growth factor from vascular endothelial cells. This stabilizes sirtuin 1, an essential keratinization inducer in keratinocytes, hence causing hyperkeratinization.
HFSR typically appears within the first 2 to 4 weeks of treatment initiation, with severity also likely to peak early.7,14,15 HFSR clinical presentation can be divided into several phases. In the prodromal phase, the patient notices dysesthesia, which progresses over several days to burning pain; palms and soles become symmetrically erythematous and edematous. The inflammatory phase follows: A well-defined symmetrical erythema and tense bullae appear on the palms and soles. The hyperkeratotic phase is next: Localized well-demarcated painful yellowish hyperkeratotic patches overlie erythematous base pressure-bearing areas.5 The patient can experience paresthesia, dysesthesia, and pain, which may be out of proportion to the clinical appearance of the lesions. Finally, the resolution phase is characterized by excessive desquamation and fissures.1,5,16,17 Other concomitant alterations have been described, including scalp dysesthesia, angular cheilitis, perianal rashes, and facial erythema resembling seborrheic dermatitis. It is uncertain whether the pathophysiology is the same.1
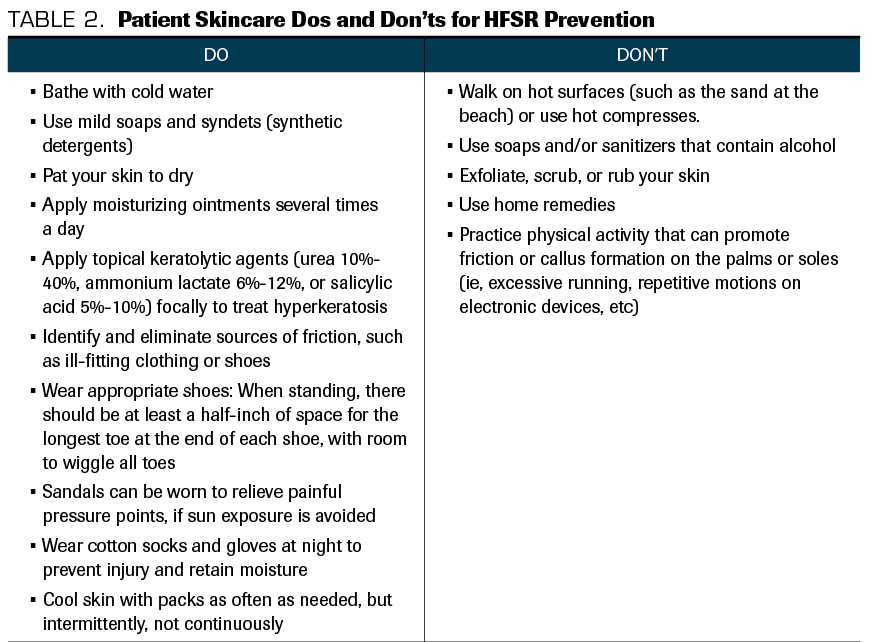
Several prophylactic measures have been described. It is recommended that before initiating treatment with a kinase-targeted therapy, a patient’s hands and feet should be examined for existing hyperkeratotic lesions and, if present, debrided by a podiatrist or dermatologist. Patients should take measures to ensure that their skin remains extremely moisturized, utilizing 10% urea cream, and to avoid friction as much as possible (summarized in Table 2), and these measures should be maintained even if HFSR occurs.4,7,9,16 Answer A is therefore incorrect: Hot compresses and scrubbing the lesions (no matter how hyperkeratotic they look) will worsen HFSR.
Table 2. Patient Skincare Dos and Don’ts for HFSR Prevention
Patients who use 10% urea cream prophylactically, 3 times a day, have lower rates of HFSR. This was observed in a randomized, open-label trial that included 871 patients with hepatocellular carcinoma being treated with sorafenib. The use of urea-based cream extended the time to the first occurrence of HFSR, and it improved patient quality of life compared with best supportive care.18 Other topical keratolytic agents (ammonium lactate 6%-12%; salicylic acid 5%-10%) can be used to treat focal hyperkeratosis. Higher concentrations of keratolytics (urea 40%) might be implemented once HFSR is established.16 In addition, celecoxib has been suggested to lower incidence rates of HFSR. In an open-label prospective study of 116 patients with advanced hepatocellular cancer, patients who received sorafenib with celecoxib achieved lower grade 2 and grade 3 HFSR incidence rates compared with those who received sorafenib alone.19 Finally, a retrospective study found that prophylactic oral dexamethasone 2 mg, taken once a day, delayed dose modifications of regorafenib and decreased the incidence of grade 3 HFSR (3.2% in the experimental population vs 25.7% in the controls).20
After establishing a diagnosis of HFSR, treatment should be individualized using the National Cancer Institute’s Common Terminology Criteria for Adverse Effects. HFSR of grade 1 is characterized by minimal changes, such as erythema, edema, or hyperkeratosis without pain, and it can be treated with topical lidocaine 2% to 6% in gel, cream, or patch form. In grade 2 HFSR, painful skin changes (eg, blisters, erythema, hyperkeratotic plaques) limit instrumental daily life activities, and grade 3 HFSR is characterized by severe skin changes (eg, moist desquamation, ulceration, blistering, fissures, or local injury causing infectious complications) and pain that limits self-care activities of daily life.5,16,21 Treatment of grade 2 and 3 HFSR involves pain control with systemic medications, including nonsteroidal anti-inflammatory drugs and opioids or γ-aminobutyric acid antagonists.7,17
Superpotent topical steroids, such as clobetasol propionate ointment 0.05% or fluocinonide 0.05%, are recommended for treatment of the inflamed, erythematous areas.7,15 Topical steroids induce vasoconstriction, promote antimitotic actions, and have anti-inflammatory activity.22 No studies have compared the different strengths of topical steroids in HFSR. Superpotent ones are usually recommended since the percutaneous absorption in hyperkeratotic or lichenified lesions on the palms and soles is diminished.23,24 Clobetasol, due to its superior potency, has also proved effective in treating “steroid-resistant dermatosis.”25 The vehicle also matters; an ointment base, not cream, is preferred for infiltrated, lichenified lesions. An ointment’s occlusive effect increases the hydration of the stratum corneum, enhancing the drug’s penetration.26-28 Answer B is therefore incorrect: Hydrocortisone 1% cream is a low-potency steroid. Steroids are an adjuvant to and not a substitute for the aforementioned supportive measures. Answer C is therefore incorrect: Superpotent steroids are not recommended as HFSR monotherapy.
Other translational studies have suggested treatment with sildenafil, heparin-containing ointments,29 and narrowband UV-B phototherapy,23 but more research is needed.15 In a randomized multicenter phase 2 trial, patients with metastatic renal cell carcinoma who were being treated with sorafenib found that hydrocolloid dressing containing ceramide improved symptom management of HFSR.24 An ongoing phase 3 randomized trial is investigating this therapy.30 Several other therapy trials with topical sildenafil (NCT03229512), tazarotene (NCT04071756), and nicotinamide (NCT04242927) are ongoing.
The option of holding MKI, as a measure to improve HFSR, should be reserved for severe cases (grade 3) or refractoriness to supportive measures. When holding is needed, most HFSR treatment algorithms recommend holding MKI for 7 days (or until HFSR symptoms resolve), then resuming at a lower dose. If HFSR worsens or does not improve, dose interruption or discontinuation is suggested. If severe HFSR recurs despite supportive measures, therapy should be discontinued.7 Answer E is therefore incorrect: For grade 2 HFSR, try supportive measures before discontinuing the MKI. D, then, is the best answer.
Prognosis for patients with HFSR is good, since this AE is reversible and treatable. It has been suggested that HFSR occurrence is a predictor of prolonged survival. In individuals with renal cell carcinoma treated with sunitinib and sorafenib, HFSR is associated with significantly better progression-free survival (PFS).7,16 A similar observation was made in patients with metastatic colorectal cancer treated with regorafenib who experienced severe HFSR.31
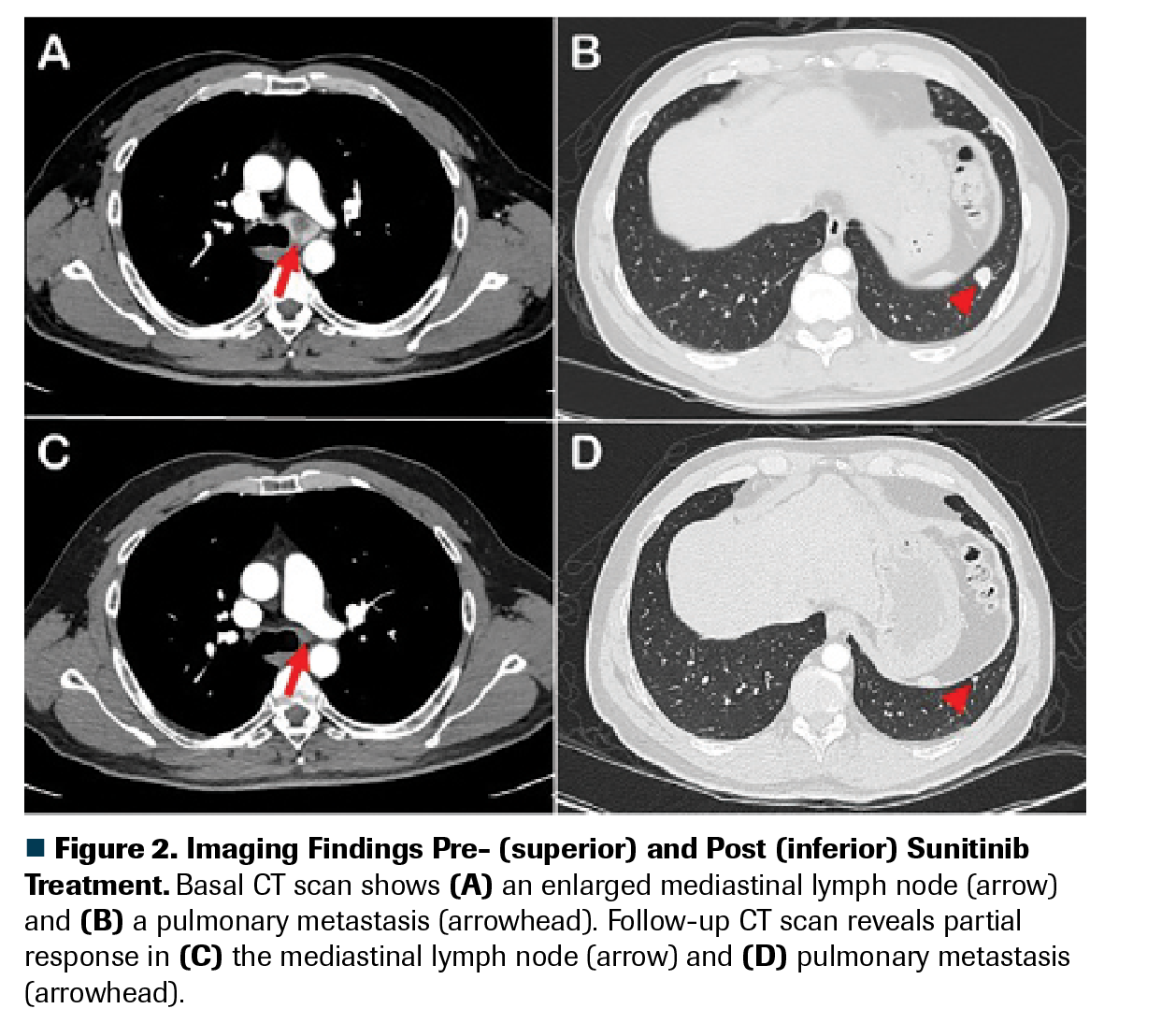
Figure 2. Imaging Findings Pre- (superior) and Post (inferior) Sunitinib Treatment
Outcome of This Case
The patient was treated with a high-potency topical steroid (clobetasol 0.05% ointment twice daily) and a keratolytic agent (urea 40% cream once daily), which improved the symptoms to grade less than or equal to 1 in 2 weeks. Clobetasol was tapered after 2 weeks. Rechallenge courses with urea were given when hyperkeratosis relapsed. Supportive care recommendations were reinforced. Sunitinib dose did not need to be delayed or adjusted. The patient had a partial response in his first CT response evaluation, which is maintained to this day (Figure 2). He has had a PFS of 12 months to date, with no grade greater than or equal to 2 HFSR recurrence.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References
1. Lipworth AD, Robert C, Zhu AX. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology. 2009;77(5):257-271. doi:10.1159/000258880
2. Nikolaou V, Syrigos K, Saif MW. Incidence and implications of chemotherapy related hand-foot syndrome. Expert Opin Drug Saf. 2016;15(12):1625-1633. doi:10.1080/14740338.2016.1238067
3. Miller K, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71(4):787-794. doi:10.1016/j.jaad.2014.03.019
4. Espinosa Lara P, Bueno Muiño C, Doger de Spéville B, Jiménez Reyes J. Hand-foot skin reaction to regorafenib. Actas Dermosifiliogr. 2016;107(1):71-73. doi:10.1016/j.ad.2015.03.016.
5. Chanprapaph K, Rutnin S, Vachiramon V. Multikinase inhibitor-induced hand-foot skin reaction: a review of clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2016;17(4):387-402. doi:10.1007/s40257-016-0197-1
6. Li J, Gu J. Hand-foot skin reaction with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;119:50-58. doi:10.1016/j.criterveonc.2017.09.016
7. De Wit M, Boers-Doets C, Saettini A, et al. Prevention and management of adverse events related to regorafenib. Support Care Cancer. 2014;22(3):837-846. doi:10.1107/s00520-013-2085-z
8. Lee WJ, Lee JL, Chang SE, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol. 2009;161(5):1045-1051. doi:10.1111/j.1365-2133.2009.09290.x
9. Zhu Y, Zhang X, Lou X, Chen M, Luo P, He Q. Vascular endothelial growth factor (VEGF) antibody significantly increases the risk of hand-foot skin reaction to multikinase inhibitors (MKIs): a systematic literature review and meta-analysis. Clin Exp Pharmacol Physiol. 2018;45(7):659-667. doi:10.1111/1440-1681.12935
10. Belum VR, Wu S, Lacouture ME. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Invest New Drugs. 2013;31(4):1078-1086. doi:10.1007/s10637-013-997-0
11. Belum VR, Serna-Tamayo C, Wu S, Lacouture ME. Incidence and risk of hand-foot skin reaction with cabozantinib, a novel multikinase inhibitor: a meta-analysis. Clin Exp Dermatol. 2016;41(1):8-15. doi:10.1111/ced.12694
12. Ding F, Liu B, Wang Y. Risk of hand-foot skin reaction associated with vascular endothelial growth factor–tyrosine kinase inhibitors: a meta-analysis of 57 randomized controlled trials involving 24,956 patients. J Am Acad Dermatol. 2020;83(3):788-796. doi:10.1016/j.jaad.2019.04.021
13. Massey PR, Okman JS, Wilkerson J, Cowen EW. Tyrosine kinase inhibitors directed against the vascular endothelial growth factor receptor (VEGFR) have distinct cutaneous toxicity profiles: a meta-analysis and review of the literature. Support Care Cancer. 2015;23(6):1827-1835. doi:10.1007/s00520-014-2520-9
14. Wood LS, Lemont H, Jatoi A, Lacouture M, Robert C, Keating K. Practical considerations in the management of hand-foot skin reaction caused by multikinase inhibitors. Community Oncol. 2010;7(1):23-29. doi:10.1016/S1548-5315(11)70385-0
15. McLellan B, Ciardiello F, Lacouture ME, Segaert S, Van Cutsem E. Regorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and management. Ann Oncol. 2015;26(10):2017-2026. doi:10.1093/annonc/mdv244
16. Gomez P, Lacouture ME. Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer. Oncologist. 2011;16(11):1508-1519. doi:10.1634/theoncologist.2011-0115
17. Kusari A, Borok J, Han AM, Valderrama AJ, Friedlander SF. Hand-foot-skin reaction related to use of the multikinase inhibitor sorafenib and hard orthotics. Pediatr Dermatol. 2018;35(4):e206-e209. doi:10.1111.pde.13523
18. Ren ZG, Zhu KS, Kang HY, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(8):894-900. doi:10.1200/JCO.2013.52.9651
19. Chen J-C, Wang J-C, Pan Y-X, et al. Preventive effect of celecoxib in sorafenib-related hand-foot syndrome in hepatocellular carcinoma patients, a single-center , open-label , randomized , controlled clinical phase III trial. Am J Cancer Res. 2020;10(5):1467-1476.
20. Fukuoka S, Shitara K, Noguchi M, et al. Prophylactic use of oral dexamethasone to alleviate fatigue during regorafenib treatment for patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2017;16(2):e39-e44. doi:10.1016/j.clcc.2016.07.012
21. Koldenhof JJ, Witteveen PO, de Vos R, et al. Symptoms from treatment with sunitinib or sorafenib: a multicenter explorative cohort study to explore the influence of patient-reported outcomes on therapy decisions. Support Care Cancer. 2014;22(9):2371-2380. doi:10.1007/s00520-014-2223-2
22. Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1-15; quiz 16-18. doi:10.1016/j.jaad.2005.01.010
23. Hung C-T, Chiang C-P, Wu B-Y. Sorafenib-induced psoriasis and hand-foot skin reaction responded dramatically to systemic narrowband ultraviolet B phototherapy.J Dermatol. 2012;39(12):1076-1077. doi:10.1111/j.1346-8138.2012.01615.x
24. Shinohara N, Nonomura N, Eto M, et al. A randomized multicenter phase II trial on the efficacy of a hydrocolloid dressing containing ceramide with a low-friction external surface for hand-foot skin reaction caused by sorafenib in patients with renal cell carcinoma. Ann Oncol. 2014;25(2):472-476. doi:10.1093/annonc/mdt541
25. Olsen EA, Cornell RC. Topical clobetasol-17-propionate: review of its clinical efficacy and safety. J Am Acad Dermatol. 1986;15(2 Pt 1):246-255. doi:10.1016/s0190-9622(86)70164-3
26. Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13(9):1001-1011. doi:10.1634/theoncologist.2008-0131
27. Williams LA, Ginex PK, Ebanks GL Jr, et al. ONS guidelinesTM for cancer treatment-related skin toxicity. Oncol Nurs Forum. 2020;47(5):539-556. doi:10.1188/20.ONF.539-556
28. Ai L, Xu Z, Yang B, He Q, Luo P. Sorafenib-associated hand-foot skin reaction: practical advice on diagnosis, mechanism, prevention, and management. Expert Rev Clin Pharmacol. 2019;12(12):1121-1127. doi:10.1080/17512433.2019.1689122
29. Li JR, Yang CR, Cheng CL, et al. Efficacy of a protocol including heparin ointment for treatment of multikinase inhibitor-induced hand-foot skin reactions. Support Care Cancer. 2013;21(3):907-911. doi:10.1007/s00520-012-1693-3
30. Zenda S, Ryu A, Takashima A, et al. Hydrocolloid dressing as a prophylactic use for hand-foot skin reaction induced by multitargeted kinase inhibitors: protocol of a phase 3 randomised self-controlled study. BMJ Open. 2020;10(10):e038276. doi:10.1136/bmjopen-2020-038276
31. Kobayashi K, Kawakami K, Yokokawa T, et al. Association of hand-foot skin reaction with regorafenib efficacy in the treatment of metastatic colorectal cancer. Oncology. 2019;96(4):200-206. doi:10.1159/000495989
