Have the Changes in Treatment of Rectal Cancer Made a Significant Difference to Our Patients?
Treatment for patients with locally advanced, resectable rectal cancer has clearly evolved, with significant refinements in preoperative assessment, surgical technique, and use of preoperative chemoradiation.
The treatment for patients with locally advanced, resectable rectal cancer has evolved over the years. Various combinations and sequences of chemotherapy, radiation therapy, and total mesorectal excision (TME)-based surgery are the mainstay of current therapy. Preoperative combined chemoradiation, followed by surgery, is now the preferred treatment strategy, with the majority of patients receiving either infusion fluorouracil (5-FU) or capecitabine (Xeloda) with radiation. Clinical trials with oxaliplatin (Eloxatin)-based neoadjuvant chemoradiation have not shown improvement in the pathologic complete response rate (pCR) compared with 5-FU; however, final data addressing local recurrence rates and disease-free survival are pending. The use of adjuvant chemotherapy following preoperative chemoradiation and surgery has not been optimally defined. Some studies have shown that patients who obtained significant pathologic downstaging after chemoradiation and surgery have improved survival with the use of adjuvant chemotherapy. Since FOLFOX (folinic acid, 5-FU, and oxaliplatin) is the preferred adjuvant chemotherapy regimen for stage III colon cancer based on randomized clinical trial results, FOLFOX is also recommended for rectal cancer patients as an adjuvant therapy approach.
Introduction
The most significant advances in surgical management of the rectal cancer patient in the last three decades have come about because of an enhanced appreciation of the importance of patterns of cancer spread,[1] anatomical planes,[2] neural innervations,[3] and negative surgical margins.[4-8] This has led to development of surgical approaches, specifically total mesorectal excision (TME) and autonomic nerve preservation (ANP), that have resulted in improved local control as well as improved functional results.[9-11] In addition, because of advances in imaging and pathologic assessment, we have an improved ability to identify preoperatively the need for neoadjuvant therapy and postoperatively the need for adjuvant therapy. This has facilitated our ability to better tailor therapy for the individual patient and determine the sequence of treatments, namely surgery up front with post-operative adjuvant therapy as pathologically determined vs neoadjuvant therapy based on pretherapy assessment including physical exam, histology, and imaging. Clearly, the goal and challenge has been and continues to be optimal treatment rather than undertreatment or overtreatment of a particular rectal cancer patient.
Surgical Technique and Pathologic Assessment
The technique of operating in embryologic planes between the fascia propria of the mesorectum (which envelops the mesorectum and mesorectal lymph nodes) and the parietal fascia (which overlies important pelvic structures such as autonomic nerves) is referred to as sharp mesorectal excision. It decreases the likelihood of a positive circumferential margin (CRM), shown to be a predictor of local recurrence and poor survival,[1,4-8,12] and is used in performing a TME. Subsequent to TME's popularization,[9] introduction of TME techniques into surgical training programs led to a decrease in local recurrence and improved survival in several European populations.[13-15] In conjunction with ANP, sharp mesorectal excision has also led to decreased rates of postoperative genitourinary dysfunction.[10]
Parallel to advances in surgical technique, there have been advances in pathologic grading of adequacy of rectal cancer resection into the mesorectal plane (a good plane of surgery achieved), intramesorectal plane (a moderate plane of surgery achieved), and lastly the muscularis propria plane (a poor plane of surgery achieved, based on grading of the resected specimen).[16] The plane of surgery achieved is an important prognostic factor, given that surgery in the mesorectal plane lowers local recurrence rates.[2,16] This finding further underscores the important role of optimal surgical technique.
Although laparoscopic surgery for colon cancer is now well accepted, with proven oncologic equivalency and superior earlier postoperative recovery relative to open surgery, its acceptance in rectal cancer surgery has not been universal, in part because of isolated reports documenting higher but nonsignificant rates of CRM involvement[17] and a trend toward worse male sexual function following a laparoscopic anterior resection.[18] However, despite differences in CRM rates, 3- and 5-year follow-up have not demonstrated a difference in local and distant recurrence, nor in overall or disease-free survival rates, between laparoscopic resection and open rectal cancer resection.[18,19] Clearly, larger sample studies with longer follow-up are needed to fully address this important issue. Nevertheless, at the present time, although there might be short-term postoperative benefits to laparoscopic rectal cancer resection, an oncologic benefit has yet to be demonstrated. However, because of the earlier postoperative recovery with the laparoscopic approach, it is conceivable that a laparoscopic resection may lead to an increase in patient compliance with postoperative adjuvant therapies.
Despite advances in surgical technique, there are cases of locally advanced rectal cancer in which surgery as the sole treatment may lead to a positive CRM, resulting in an increased risk of local failure and a poorer survival outcome. These include fixed cancers invading local structures and abutting pelvic sidewalls. In these cases, neoadjuvant therapy, in the form of chemoradiation (CMT), chemotherapy, or radiation alone (as reviewed later in this article), is indicated in an attempt to shrink the bulk of disease and enhance the likelihood of obtaining a negative CRM while preserving anal sphincters. The bigger challenge is determining the need for neoadjuvant therapy for disease that is nonfixed yet at increased risk of local failure, such as node-positive (N+) disease or disease involving/abutting the mesorectal fascia. Conventional treatment for locally advanced, clinically resectable (T3 and/or N+) rectal cancer is neoadjuvant CMT. However, our current ability to accurately identify N+ disease is limited, leading to overtreatment of perhaps 18% of patients, as shown in the German CAO/ARO/AIO-94 trial,[20] or undertreatment of 20% to 30% of N+ rectal cancer, as shown by a consortium of high-volume centers.[21] Contrast-enhanced MRI shows promise, with reported improved sensitivity and specificity for detection of metastatic lymph nodes.[22]
Similarly, preoperative high-resolution MRI and expert radiological interpretation may help oncologists select rectal cancer patients likely to have a good outcome with surgery alone.[23-25] However, there are a number of limiting factors-including the ability of high-resolution MRI to determine the proximity of the rectal cancer to the mesorectal fascia, the likelihood of positive CRM, and the need for downstaging with preoperative CMT; in addition, a positive CRM is not always reliably predicted, and preoperative CMT is sometimes erroneously omitted.[25] Neoadjuvant systemic chemotherapy alone has been explored, with promising results as will be discussed.
Neoadjuvant Chemoradiation, Radiation, or Chemotherapy Alone?
The conventional treatment for locally advanced, clinically resectable (T3 and/or N+) rectal cancer is preoperative CMT. When 5-FU is used concurrently with radiation, continuous infusion (CI) of capecitabine (Xeloda) is the conventional treatment.[26,27] The National Surgical Adjuvant Breast and Bowel Project (NSABP) R-04 trial compared preoperative CMT with CI 5-FU vs capecitabine (with or without oxaliplatin [Eloxatin]). Compared to those treated with CI 5-FU, patients randomized to capecitabine had similar rates of pathologic complete response (pCR, 22% vs 19%, respectively), sphincter-sparing surgery (63% vs 61%, respectively), and grade 3+ diarrhea (11%).[28]
Local recurrences can occur late in rectal cancer. Patients who receive postoperative CMT have an increase in local recurrence and a decrease in survival after 5 years. The Intergroup postoperative rectal adjuvant trial INT 0114 in patients with T3-4 and N+ disease showed rates of local control and survival continue to decrease beyond 5 years.[29] At 7 years, the local recurrence rate was 17% and survival was 56%, vs 14% and 64%, respectively, at 5 years. In contrast, in patients who received preoperative CMT in the German CAO/ARO/AIO-94 trial there was a decrease in survival at 10 years vs 5 years (74% vs 60%) but no change in local recurrence (6%).[30]
The two randomized trials of preoperative vs postoperative CMT for clinically resectable T3-4 Nany rectal cancer (NSABP R0-3)[31] and the German CAO/ARO/AIO-94 trial[20] reported opposite results. In the German trial, patients who received preoperative therapy had a significant decrease in local recurrence (6% vs 15%, P = .006), acute toxicity (27% vs 40%, P = .001), and chronic toxicity (14% vs 24%, P = .012), and among 194 patients judged by the surgeon pretreatment to require abdominoperineal resection, there was a significant increase in sphincter preservation (39% with preoperative therapy vs 20% with postoperative treatment, P = .004). There was no difference in survival (74% vs 76%, respectively). The results were updated with a median follow-up of 11.2 years. At 10 years the local control benefit of preoperative therapy was still superior to that of postoperative therapy (cumulative incidence of local relapse, 6% vs 10%, respectively), and the survival was equivalent (60% for both groups).[30]
Compared with postoperative therapy, patients who received preoperative therapy in the NSABP R-03 trial had a significant improvement in 5-year disease-free survival (65% vs 53%, P = .011) and a borderline significant improvement in 5-year overall survival (75% vs 66%, P = .065).[31] There was no difference in 5-year local recurrence (11% for both groups). There was a corresponding higher incidence of grade 4+ toxicity (33% vs 23%), but grade 3+ toxicity was lower (41% vs 50%). Lastly, based on a prospective office assessment by the operating surgeon, there was no improvement in sphincter preservation (48% vs 39%).
Results of the NSABP trial should be interpreted with caution since only 267 of the 900 planned patients were accrued, thereby limiting the statistical power to detect differences. The German trial met its accrual goals, and based on the positive results, preoperative CMT remains the standard of care.
Preoperative 5 Gy × 5 or chemoradiation?
There are two randomized trials of short-course radiation vs CMT. Bujko et al randomized 316 patients with cT3 rectal cancer.[32,33] All tumors were above the anorectal ring, TME was performed for distal tumors, and there was no radiation quality-control review. There were no significant differences in local control or survival. The incidence of positive radial margins was lower following CMT than with short-course radiation alone (4% vs 13%, P = .017). A similar trial was reported by Ngan et al.[34] A total of 326 patients with T3Nany rectal cancer (56% were N0) were randomized. In contrast to the trial by Bujko et al, patients received postoperative adjuvant chemotherapy. There were no significant differences in 3-year local recurrence (8% vs 4%) or 5-year survival (74% vs 70%).
These trials challenge the role of long-course CMT in selected patients, but they need to be examined in perspective. Neither was limited to N+ disease, and both require longer follow-up.
Selective use of radiation
Given the improvements in systemic chemotherapy, there may be an opportunity to use preoperative radiation more selectively. In a prospective trial reported in abstract form, Cercek et al treated 32 patients with ultrasound-staged uT2N1 or uT3N0-1 rectal cancer by preoperative assessment who were treated with neoadjuvant FOLFOX (folinic acid, 5-FU, and oxaliplatin) + bevacizumab (Avastin).[35] Pelvic radiation was reserved for patients who progressed preoperatively or who following surgery had either pT4, pN2, or positive margins. Of the 30 patients who underwent low anterior resection, none required radiation, the pCR rate was 27%, and 2 required postoperative radiation. This approach remains investigational and will be examined in an Intergroup phase II/III trial.
Chemoradiation regimens
New agents. Both cytotoxic and targeted chemotherapeutic agents have been incorporated into phase I/II preoperative CMT regimens. Most of the regimens report higher pCR rates compared with 5-FU alone.
Four randomized trials have examined the impact that adding oxaliplatin to 5-FU- or capecitabine-based CMT has on response rates and acute toxicity in patients with cT3-4 and/or N+ rectal cancer. The STAR (Studio Terapia Adiuvante Retto)-01 trial randomized 747 patients to preoperative CMT with 50.4 Gy plus CI 5-FU with or without oxaliplatin.[36] There was a significant increase in grade 3+ toxicity with oxaliplatin (24% vs 8%, P < .001), with no improvement in pCR (15% vs 16%).
In the ACCORD (Action Clinique Coordonnes en cancrologie Digestive) trial, 598 patients were randomized to preoperative CMT with 50 Gy plus capecitabine plus oxaliplatin (CAPOX) vs 45 Gy plus capecitabine.[37] There was a similar significant increase in grade 3+ toxicity with oxaliplatin (25% vs 11%, P < .001), with no improvement in pCR (19% vs 14%).
NSABP R-04 was a four-arm investigation (2 × 2 comparison) of CI 5-FU vs capecitabine-based preoperative CMT (50.4 Gy) with or without oxaliplatin.[28] A total of 1606 patients with cT3 and/or N+ disease were randomized. Addition of oxaliplatin (to either 5-FU or capecitabine) was associated with significantly more grade 3+ diarrhea (15% vs 7%, P = .0001), with no improvement in pCR (21% vs 19%) or sphincter-sparing surgery (60% vs 64%).
The German CAO/ARO/AIO-04 trial randomized 1265 patients with cT3-4 and/or N+ disease to the pre-operative arm of CAO/ARO/AIO-94 (50.4 Gy + 5-FU) vs 50.4 Gy/5-FU + oxaliplatin (at 50 mg/m2 weekly).[38] In contrast with the STAR-01, ACCORD, and NSABP R-04 trials, patients who received oxaliplatin-based CMT had a significant improvement in pCR (17% vs 13%, P = .045), with no corresponding increase in acute grade 3+ toxicity (23% vs 22%). The results of a similar trial (PETACC-6) are pending.
Since three of four randomized trials reveal an increase in acute toxicity with no benefit in the pCR rate, the current standard is not to include oxaliplatin in preoperative CMT regimens. Data from these trials on local control and survival are not available, however, and this recommendation may need to be modified once these data are reported.
The role of targeted biological agents such as bevacizumab is being tested. Preliminary phase I trials using pre-operative CMT with CAPOX plus bevacizumab have reported pCR rates of 18% to 24%.[39,40] However, given the lack of a survival benefit in the NSABP C-08 adjuvant colon cancer trial,[41] the ultimate role of bevacizumab in rectal cancer therapy remains unclear.
Although the report from Heidelberg of capecitabine–irinotecan (CAPEIRI)-based CMT cited a pCR rate of 25%,[42] other trials with 5-FU, capecitabine, or CAPOX reported more limited rates of 5% to 12%.[43,44] It is unknown whether the benefit of patient selection based on K-ras expression seen in patients with metastatic disease will be helpful in the adjuvant setting for patients with rectal cancer.[40]
TABLE
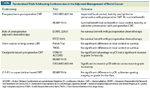
Randomized Trials Addressing Controversies in the Adjuvant Management of Rectal Cancer
Induction chemotherapy. The Spanish Granada Cancer Registry (GCR)-3 randomized phase II trial compared induction chemotherapy followed by CMT with conventional preoperative CMT followed by surgery and postoperative chemotherapy.[45] A total of 108 patients received 50.4 Gy plus CAPOX preoperatively and were randomized to receive 4 months of CAPOX either by induction or adjuvant (postoperative) therapy. Although the pCR rates were not different (14% vs 13%), grade 3+ toxicity was lower (17% vs 51%, respectively, P = .00004) and the ability to receive all four chemotherapy cycles was higher (93% vs 51%, P = .0001) with induction.
Radiation techniques and dose
The clinical utility of routine 3-D and intensity-modulated radiation therapy (IMRT) planning techniques is being investigated.[46,47] The most important contributions of 3-D treatment planning are the ability to plan and localize the target and normal tissues at all levels of the treatment volume and to obtain dose-volume histogram data. An analysis of 3-D treatment planning techniques suggests that the volume of small bowel in the radiation field is decreased with protons as compared with photons.[48] IMRT treatment planning techniques can further decrease the volume of small bowel in the field,[49] but the clinical benefit of IMRT compared with 3-D or conventional treatment delivery remains to be determined.[47]Guidelines for the definition and delineation of the clinical target volumes are available from a number of investigators.[50,51]
The Radiation Therapy Oncology Group (RTOG) R-0012 phase II randomized trial compared twice-a-day preoperative CMT up to 60 Gy (1.2 Gy to 45.6 Gy, with a boost of 9.6 Gy to 14.4 Gy) with conventional fractionation (1.8 Gy to 45 Gy, with a boost of 5.4 Gy to 9.0 Gy) plus 5-FU/irinotecan.[52] Both regimens resulted in a 28% pCR rate but were also associated with a greater than 40% rate of grade 3/4 acute toxicity.
Adjuvant chemotherapy
Historically, adjuvant chemotherapy was administered in combination with CMT after surgical resection for stage II and III rectal adenocarcinoma. The introduction of preoperative CMT for clinical stage II and III rectal cancer as the preferred standard of care has resulted in variation in the use of adjuvant chemotherapy after neoadjuvant CMT and surgery. Some potential reasons for failure to administer adjuvant chemotherapy include uncertainty as to whether adjuvant chemotherapy is necessary for patients who demonstrated a pCR after neoadjuvant CMT and concerns that patients who have undergone CMT and surgery may not be able to tolerate additional therapy after surgery. A question of significant importance is whether the relatively brief exposure to chemotherapy in combination with preoperative radiation therapy (which generally includes infusion 5-FU or capecitabine) is truly sufficient to improve survival, particularly for high-risk rectal cancer patients. Survival rates in rectal cancer are a persistent problem; only about one-third of patients with stage IIC and IIIC rectal cancer, for example, survive 5 years.[53] The 5-year-survival rates of stage II A (64.5%) and IIB (51.6%) rectal cancer are also suboptimal. An extrapolation from colon cancer clinical trials would strongly suggest that approximately 6 months of adjuvant chemotherapy with FOLFOX is the optimal current strategy to improve survival for individuals at risk for recurrence (eg, those with stage III colon cancer). Unfortunately, two randomized National Cancer Institute Gastrointestinal Intergroup stage II/III rectal cancer adjuvant clinical trials (E3201, E5204) closed prematurely because of insufficient accrual. As similar trials mature, however, they will provide some information about toxicity of combination therapy, sites of recurrence, and progression-free survival, as well as limited data on overall survival.
Two randomized trials (Table) address whether postoperative chemotherapy is beneficial following preoperative therapy. The European Organisation for Research and Treatment of Cancer (EORTC) trial 22921 was a four-arm randomized study of 1011 patients who received 45 Gy preoperatively, with or without concurrent bolus 5-FU/leucovorin, followed by surgery with or without 4 cycles of postoperative 5-FU/leucovorin.[54] Only 37% of patients had a TME. The FFCD (Fdration Francophone de la Cancrologie Digestive) 9203 was a two-arm trial of 742 patients randomized to preoperative 45 Gy with or without concurrent bolus 5-FU/leucovorin.[55] However, all patients were scheduled to receive postoperative chemotherapy and 73% did receive it.
The EORTC trial demonstrated a significant decrease in local recurrence among patients who received CMT vs radiation (8% to 10% vs 17%, P < .001) but no difference in 5-year survival (65%). Only 43% of patients received 95% or more of the planned postoperative chemotherapy, however, which may explain the negative results. Furthermore, a subset analysis of the EORTC trial revealed that patients who responded to preoperative CMT had a survival benefit from postoperative chemotherapy.[56] This retrospective analysis included the group of 785 patients with cT3-4 tumors who underwent a R0 resection; only those who had tumor downstaging to ypT0-2 disease demonstrated improved 5-year disease-free survival (76.7% vs 65.6%, respectively; P = .013).[56] The FFCD trial reported a similar decrease in local recurrence (8% vs 17%, P < .05) and a corresponding increase in pCR (11% vs 4%, P < .05) with preoperative CMT, but no survival benefit (68% vs 67%).
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Bevacizumab (Avastin)
Capecitabine (Xeloda)
Fluorouracil (5-FU)
Folinic acid (leucovorin)
Irinotecan
Oxaliplatin (Eloxatin)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
A pooled analysis of the two trials with a median follow-up of 5.6 years confirmed that patients who received preoperative CMT vs radiation had a significant decrease in local recurrence (11% vs 15%, P = .0001), however there was no difference in 5-year overall survival (66%) for both groups).[57] Other retrospective analyses have indicated that patients who experience pathologic downstaging after treatment with neoadjuvant CMT and surgery were most likely to receive additional benefit from adjuvant chemotherapy.[58-60] For example, two separate retrospective series from MD Anderson including clinical stage II or III rectal cancer patients (117 and 407 patients, respectively) who underwent R0 resection showed improved disease-free survival if significant downstaging was noted and patients received adjuvant chemotherapy.[58,59] A small Canadian retrospective study also showed that those achieving significant downstaging with neoadjuvant CMT had improved progression-free and cause-specific survival.[60]
These results need to be placed in perspective. Given that most patients did not receive adequate doses of postoperative chemotherapy in the EORTC trial, and the FFCD trial only tested the impact of concurrent chemotherapy with preoperative radiation, preoperative CMT followed by surgery and 4 months of postoperative adjuvant chemotherapy remains the standard practice in North America. However, there is still considerable controversy in some European countries regarding the use of postoperative adjuvant chemotherapy.[61]
As mentioned, adjuvant oxaliplatin-based chemotherapy for patients with stage III colon cancer has resulted in significant improvement in overall survival. Currently, no prospective data are available on patients with stage II and III rectal cancer, to determine the efficacy of adjuvant oxaliplatin combination chemotherapy. Two Intergroup randomized clinical trials that included adjuvant FOLFOX chemotherapy closed prematurely because of lack of accrual (E3201, E5204), and a third phase II study by the Eastern Cooperative Oncology Group (ECOG) does not have mature data at this time (E3204). The German Rectal Cancer Study Group recently reported safety data from their randomized phase III rectal cancer study of 1265 patients (CAO/ARO/AIO-04), comparing neoadjuvant CMT, surgery, and adjuvant 5-FU vs neoadjuvant CMT with 5-FU and oxaliplatin, surgery, and FOLFOX adjuvant chemotherapy.[62] This study is designed to demonstrate whether oxaliplatin-based chemotherapy will improve 3-year disease-free survival.
Most investigators believe that it is reasonable to use the same chemotherapy for adjuvant treatment of colon and rectal cancer. For patients selected to receive postoperative adjuvant chemotherapy, treatment with 4 to 6 months (8 to 12 cycles) of mFOLFOX6 is recommended.
Summary
Treatment for patients with locally advanced, resectable rectal cancer has clearly evolved, with significant refinements in preoperative assessment, surgical technique, and use of preoperative CMT. These have improved patient outcomes, including fewer patients requiring a permanent colostomy, preservation of postoperative and sexual function leading to improved quality of life, and declining incidence of local recurrence. Care of patients with locally advanced, resectable rectal cancer must be further optimized. Important issues include the need for surgical and radiation quality control, increasing the use of TME, proper evaluation and careful implementation of emerging surgical technology, decreasing the rate of long-term sequelae of CMT (eg, sexual dysfunction), defining the optimal radiation schedule for individual patients (eg, short course vs long course), defining a select population of patients who may not require radiation, determining the significance of pCR on outcome and integration of adjuvant therapy, and investigating adjuvant therapy strategies to improve the overall survival of rectal cancer patients.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996-9.
2. Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821-8.
3. Lee JF, Maurer VM, Block GE. Anatomic relations of pelvic autonomic nerves to pelvic operations. Arch Surg. 1973;107:324-8.
4. Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235:449-57.
5. Cawthorn SJ, Parums DV, Gibbs NM, et al. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer. Lancet. 1990;335:1055-9.
6. Wibe A, Rendedal PR, Svensson E, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89:327-34.
7. Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303-12.
8. Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707-11.
9. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-82.
10. Enker WE. Potency, cure, and local control in the operative treatment of rectal cancer. Arch Surg. 1992;127:1396-401.
11. Guillem JG, Lee-Kong SA. Autonomic nerve preservation during rectal cancer resection. J Gastrointest Surg. 2010;14:416-22.
12. Bernstein TE, Endreseth BH, Romundstad P, Wibe A. Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg. 2009;96:1348-57.
13. Wibe A, Moller B, Norstein J, et al. A national strategic change in treatment policy for rectal cancer--implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum. 2002;45:857-66.
14. Martling A, Holm T, Rutqvist LE, et al. Impact of a surgical training programme on rectal cancer outcomes in Stockholm. Br J Surg. 2005;92:225-9.
15. Kapiteijn E, Putter H, van de Velde CJ. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 2002;89:1142-9.
16. Nagtegaal ID, van de Velde CJ, van der Worp E, et al. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729-34.
17. Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-26.
18. Jayne DG, Brown JM, Thorpe H, et al. Bladder and sexual function following resection for rectal cancer in a randomized clinical trial of laparoscopic versus open technique. Br J Surg. 2005;92:1124-32.
19. Jayne DG, Thorpe HC, Copeland J, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638-45.
20. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-40.
21. Guillem JG, Diaz-Gonzalez JA, Minsky BD, et al. cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol. 2008;26:368-73.
22. Lambregts DM, Beets GL, Maas M, et al. Accuracy of gadofosveset-enhanced MRI for nodal staging and restaging in rectal cancer. Ann Surg. 2011;253:539-45.
23. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779.
24. MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132-9.
25. Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711-9.
26. Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709-15.
27. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-704.
28. Roh MS, Yothers GA, O’Connell MJ, al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. J Clin Oncol. 2011;29(suppl): abstract 3503.
29. Tepper JE, O’Connell M, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control--final report of intergroup 0114. J Clin Oncol. 2002;20:1744-50.
30. Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2011;29(suppl): abstract 3516.
31. Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124-30.
32. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215-23.
33. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15-24.
34. Ngan S, Fisher R, Goldstein D, et al. A randomized trial comparing local recurrence (LR) rates between short course (SC) and long course (LC) radiotherapy (RT) for clinical T3 rectal cancer: an Intergroup trial (TROG, AGITG, CSSANZ, RACS). J Clin Oncol. 2010;28(15S): abstract 3509.
35. Cercek A, Weiser MR, Goodman KA, et al. Complete pathologic response in the primary of rectal or colon cancer treated with FOLFOX without radiation. J Clin Oncol. 2010;28(15S): abstract 3649.
36. Aschele C, Pinto C, Cordio S, et al. Preoperative fluorouracil (FU) based chemoradiation with and without weekly oxaliplatin in locally advanced rectal cancer: pathologic response analysis of the Studio Terapia Adjuvante Retto (STAR)-01 randomized phase III trial. J Clin Oncol. 2009;27(18S); abstract CRA4008.
37. Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638-44.
38. Roedel C, Becker H, Fietkau R, et al. E. Preoperative chemoradiotherapy and postoperative chemotherapy with 5-fluorouracil and oxaliplatin versus 5-fluorouracil alone in locally advanced rectal cancer: first results of the German CAO/ARO/AIO-04 randomized phase III trial. J Clin Oncol. 2011;29(suppl): abstract LBA3505.
39. Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;
27:3020-6.
40. Van Cutsem E, Lang I, D’haens G, al. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: the CRYSTAL experience. J Clin Oncol. 2008;26(15S): abstract 2.
41. Allegra CJ, Yothers G, O’Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11-6.
42. Das P, Lin EH, Bhatia S, et al. Preoperative chemoradiotherapy with capecitabine versus protracted infusion 5-fluorouracil for rectal cancer: a matched-pair analysis. Int J Radiat Oncol Biol Phys. 2006;66:1378-83.
43. Chung KY, Minsky B, Schrag D, et al. Phase I trial of preoperative cetuximab with concurrent contiuous infusion 5-fluorouracil and pelvic radiation in patients with local-regionally advanced rectal cancer. J Clin Oncol. 2006;24(18S): abstract 3560.
44. Rodel C, Arnold D, Hipp M, et al. Phase I-II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:1081-6.
45. Fernandez-Martos C, Aparicio J, Salud A, et al. Multicenter randomized phase II study of chemoradiation (CRT) followed by surgery (S) and chemotherapy (CT) versus induction chemotherapy followed by CRT and S in high-risk rectal cancer: GCR-3 final efficacy and safety results. J Clin Oncol. 2009;27(15S): abstract 4103.
46. Meyer J, Czito B, Yin FF, Willett C. Advanced radiation therapy technologies in the treatment of rectal and anal cancer: intensity-modulated photon therapy and proton therapy. Clin Colorectal Cancer. 2007; 6:348-56.
47. Aristu JJ, Arbea L, Rodriguez J, et al. Phase I-II trial of concurrent capecitabine and oxaliplatin with preoperative intensity-modulated radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2008;71:748-55.
48. Tatsuzaki H, Urie MM, Willett CG. 3-D comparative study of proton vs x-ray radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 1992;22:369-74.
49. Callister MD, Ezzell GA, Gunderson LL, et al. IMRT reduces the dose to small bowel and other pelvic organs in the preoperative treatment of rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66(suppl):S290.
50. Roels S, Duthoy W, Haustermans K, et al. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65:1129-42.
51. Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a Radiation Therapy Oncology Group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824-30.
52. Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol. 2006;24:650-5.
53. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-4.
54. Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-23.
55. Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-5.
56. Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379-86.
57. Bonnetain F, Bosset J, Gerard J, et al. An analysis of preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal cancer on survival in a pooled analysis of EORTC 22921 and FFCD 9203 trials: surrogacy in question? J Clin Oncol. 2011;29(suppl): abstract 3506.
58. Janjan NA, Crane C, Feig BW, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24:107-12.
59. Das P, Skibber JM, Rodriguez-Bigas MA, et al. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol. 2006;29:219-24.
60. Chan AK, Wong AO, Langevin J, et al. Preoperative chemotherapy and pelvic radiation for tethered or fixed rectal cancer: a phase II dose escalation study. Int J Radiat Oncol Biol Phys. 2000;48:843-56.
61. Valentini V, Aristei C, Glimelius B, et al. Multidisciplinary rectal cancer management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother Oncol. 2009;92:148-63.
62. Roedel C, Becker H, Fietkau R, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with 5-fluorouracil and oxaliplatin versus 5-fluorouracil alone in locally advanced rectal cancer: first results of the German CAO/ARO/AIO-04 randomized phase III trial.J Clin Oncol. 2011;29(suppl): abstract LBA3505.