HER2-Positive Breast Cancer: Special Challenges and Expert Insight
This article discusses recent developments in HER2-targeted therapies and new challenges for clinicians treating patients with HER2-positive breast cancer, and includes expert insights from Adam M. Brufsky, MD, PhD, of the University of Pittsburgh Medical Center in Pittsburgh, PA, V.K. Gadi, MD, PhD, of the University of Illinois in Chicago, IL, and Sara A. Hurvitz, MD, of the David Geffen School of Medicine at the University of California Los Angeles, in Los Angeles, CA, and Neil M. Iyengar, MD, of Memorial Sloan Kettering Cancer Center in New York, NY.
Recent developments in HER2-targeted therapy present new challenges for physicians treating patients with metastatic HER2-positive (HER2+) breast cancer, including how to treat patients with brain metastases, how to properly sequence therapies and make sequencing decisions, and how to identify and manage interstitial lung disease (ILD). Experts discussed these topics in a virtual CancerNetwork® Around the Practice presentation, “HER2+ Breast Cancer: Special Challenges and Expert Insight,” held April 20, 2021, and moderated by Adam M. Brufsky, MD, PhD. Members of the audience also participated in an interactive online platform by submitting responses to polling questions that were subsequently discussed by the panelists.
Screening Asymptomatic Patients for Brain Metastases
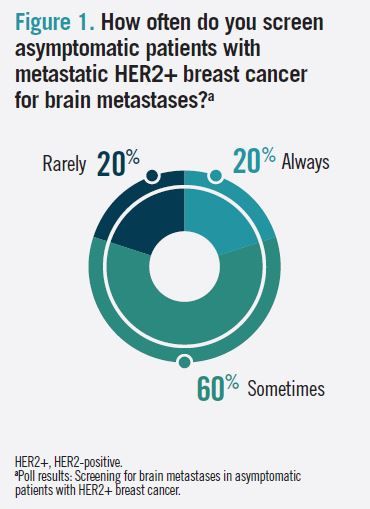
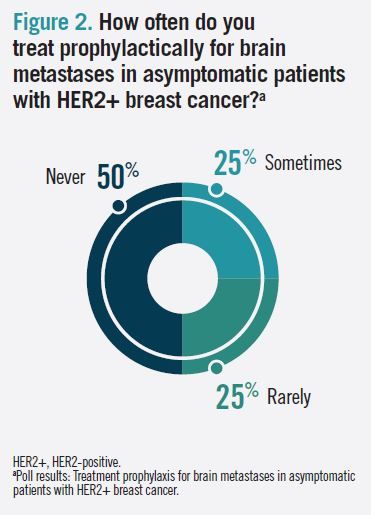
When polled about how often they screen asymptomatic patients with metastatic HER2+ breast cancer for brain metastases, 60% of audience respondents answered “sometimes” (Figure 1). In the follow-up question, “How often do you treat prophylactically for brain metastases in asymptomatic patients with HER2+ breast cancer?” 50% of respondents answered “never,” 25% answered “rarely,” and 25% answered “sometimes” (Figure 2).
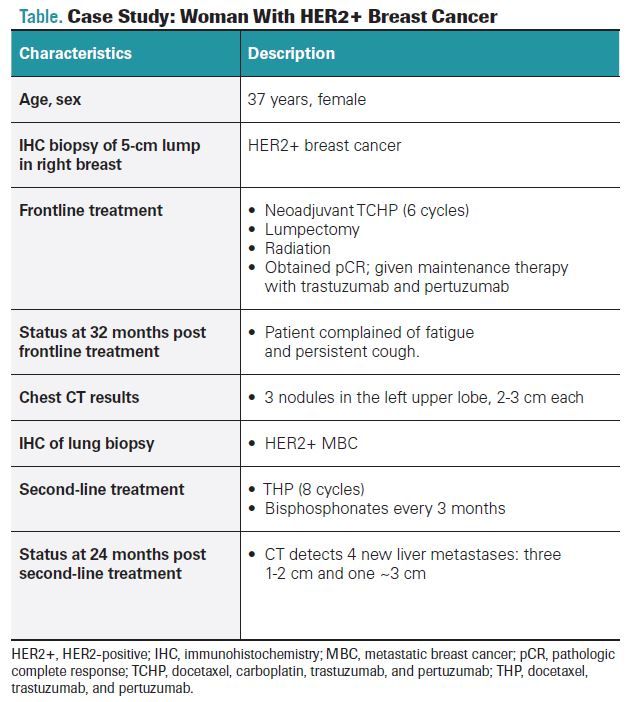
The panelists then discussed approaches to management of a 37-year-old woman with a 5-cm lump in her right breast (Table). Immunohistochemistry (IHC) showed HER2+ breast cancer. The patient obtained a pathologic complete response to 6 cycles of neoadjuvant chemotherapy (docetaxel, carboplatin, trastuzumab, and pertuzumab), lumpectomy, and radiation, followed by maintenance therapy with trastuzumab and pertuzumab. After 32 months, the patient
returned to the clinic with fatigue and persistent cough; a CT showed 3 nodules in the left upper lobe of the lung. IHC of the lung biopsy revealed HER2+ metastases, and the patient was treated with docetaxel, trastuzumab, and pertuzumab (THP) chemotherapy and given bisphosphonates every 3 months. After 24 months, a routine CT showed 4 new liver metastases.
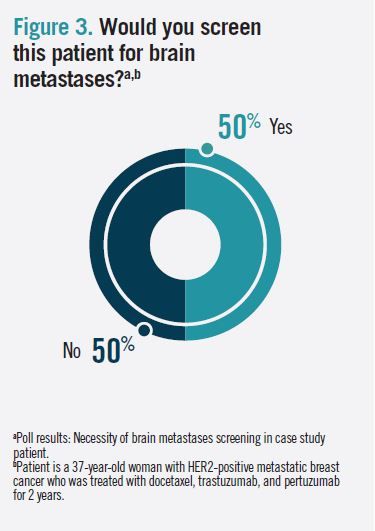
The panelists and the poll respondents were evenly divided about whether to screen this patient for brain metastases (Figure 3). The National Comprehensive Cancer Network (NCCN) guidelines recommend brain MRI with contrast for patients with recurrent or stage IV disease who have symptoms suggestive of central nervous system (CNS) metastases, and Neil M. Iyengar, MD, said that he does not routinely screen asymptomatic patients because of the lack of evidence to support a corresponding survival benefit.1
The NCCN guidelines state that clinical trials offer the best management for patients with cancer and encourage participation when possible.1 Sara A. Hurvitz, MD, recommended referring the patient for enrollment in the HER2CLIMB-02 trial (NCT03975647), a randomized, double-blind, placebo-controlled, phase 3 study to evaluate the efficacy and safety of tucatinib plus trastuzumab emtansine (T-DM1) in patients with unresectable locally advanced or metastatic HER2+ breast cancer.2 The study uses baseline brain MRI for all participants.2
V. K. Gadi, MD, PhD, said that he is increasingly inclined to screen asymptomatic patients because of the increased availability of trials for patients with CNS metastases. Hurvitz added that patient preference also influences her screening decisions.
Prophylactic Treatment of CNS Metastases
Metastases within the CNS are common among patients with HER2+ breast cancer, occurring in 25% to 50% of patients with advanced disease.3
Data from NEfERT-T (NCT00915018), a 5-year, randomized, controlled, open-label, multinational, multicenter study of 479 women with previously untreated recurrent and/or metastatic HER2+ breast cancer, suggest that HER2-targeted agents with CNS activity (such as neratinib) may reduce development of CNS metastases. The trial found that neratinib plus paclitaxel led to significantly fewer (P=.004) incidences of CNS recurrence compared with trastuzumab plus paclitaxel in patients with previously untreated, metastatic, HER2+ breast cancer (16.3% vs 31.2%; HR 0.45; 95% CI, 0.26-0.78).4
According to Gadi, the best opportunity for CNS prophylaxis is likely in the nonmetastatic setting. “In the metastatic setting, I think the cat’s out of the bag,” he said. “I don’t know how much, at this point, a brain met [metastasis] changes overall outcomes for patients.”
Sequencing of HER2-Targeted Therapies
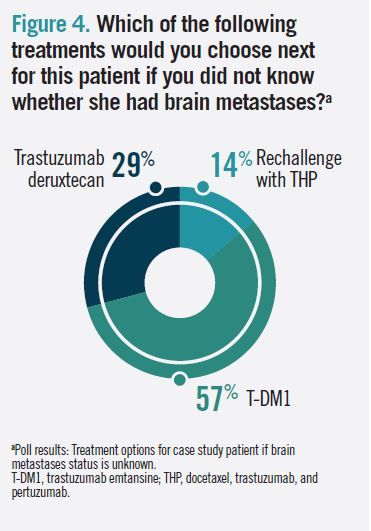
Of audience poll respondents, 57% said they would choose T-DM1 for the case patient if they did not know whether she had brain metastases (Figure 4). Gadi and Hurvitz agreed, saying current data supports this choice in a second-line setting.
If a brain MRI revealed that the case patient had two 1 cm in diameter lesions, all audience poll respondents said they would choose a regimen of tucatinib, trastuzumab, and capecitabine. The panelists agreed that tucatinib-based therapy is preferable in this scenario based on data from HER2CLIMB (NCT02614794), an international, randomized, double-blind trial comparing tucatinib plus trastuzumab and capecitabine with placebo plus trastuzumab and capecitabine in patients with HER2+ metastatic brain cancer ([MBC]; N = 612).5 The study showed that the risk of death in the overall trial population was 34% lower with addition of tucatinib to trastuzumab and capecitabine than with placebo (HR, 0.66; 95% CI, 0.50-0.88;P = .005).6 Among patients with brain metastases, the estimated progression-free survival at 1 year was 24.9% (95% CI, 16.5-34.3) and 0% in the tucatinib and placebo groups, respectively.6
“I have been fairly impressed by the combination of the systemic and CNS activity of the tucatinib-based regimen, so this is the kind of setting where I would now change treatment to tucatinib,” Iyengar said.
However, Gadi pointed out that while a tucatinib-based regimen may be optimal, patient preferences and insurance coverage are important considerations.
“If a patient says, ‘Doc, I’m not ready to take 17 pills a day and…I can’t get insurance coverage,’ [then] I think it’s important for us to talk about a simple 1-drug regimen, like T-DM1, following [stereotactic radiosurgery],” he said.
All audience poll respondents preferred T-DM1 in the second-line setting for approximately 70% of their patients. Two years after the THP treatment, the case study patient received a diagnosis of liver metastases, which were asymptomatic and accompanied by normal liver function tests. She was prescribed T-DM1 and did not receive a brain MRI.
In this situation, Iyengar said he would obtain another biopsy to confirm HER2 status and give a tucatinib-based regimen. “My practice has been largely to use [a] tucatinib-based regimen in the third-line setting, partly because of the systemic activity of that regimen but also partly because we know from the trastuzumab deruxtecan data that it is quite an active drug, even in late lines of therapy,” he said.
The tucatinib-based regimen and trastuzumab deruxtecan are both “reasonable options,” for this patient, Hurvitz said, adding that efficacy rates for trastuzumab deruxtecan are “phenomenal,” as observed in the DESTINY-Breast01 trial (NCT03248492). This phase 2, open-label, single-arm, 2-part, multicenter study of 184 patients showed a confirmed response rate of 60.9% to trastuzumab deruxtecan in patients with HER2+, unresectable and/or MBC who were previously treated with T-DM1 and received a median of 6 prior lines of therapy (n = 112; 95% CI, 53.4-68.0).7
However, 13.6% of patients in the study (n = 25) experienced trastuzumab deruxtecan-related ILD, a group of diseases (including interstitial pneumonitis) characterized by lung fibrosis and inflammation that causes breathing difficulties; 4 of these patients died.7,8
Data from the HER2CLIMB trial indicate that tucatinib is relatively safe, Hurvitz said.6 The most common adverse events observed in patients treated with tucatinib (n = 404) were diarrhea (any grade, 80.9%; grade ≥3, 12.9%), palmar-plantar erythrodysesthesia syndrome (any grade, 63.4%; grade ≥3, 13.1%), nausea (any grade, 58.4%; grade ≥3, 3.7%), fatigue (any grade, 45%; grade ≥3, 4.7%), and vomiting (any grade 35.9%; grade ≥3, 3%).6 Less than 6% of patients discontinued treatment with tucatinib because of adverse events.6
Ultimately, the panelists agreed that the patient would need to be involved in the treatment decision-making process and informed about the risks and benefits associated with each agent.
Interstitial Lung Disease
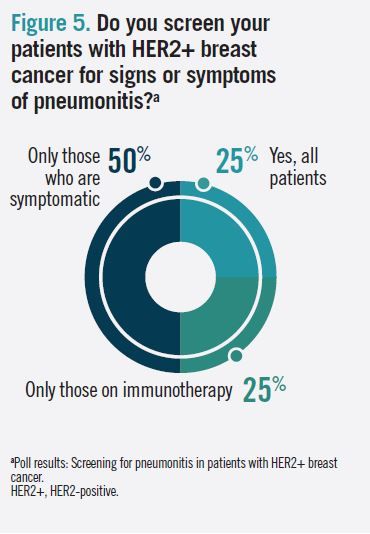
When asked whether they screen patients with HER2+ breast cancer for signs of pneumonitis, 50% of audience respondents said they screen only symptomatic patients, 25% said they screen all patients, and 25% said they screen only patients on immunotherapy (Figure 5).
The underlying mechanism of ILD is poorly understood, but the cytotoxic component of trastuzumab may be causing damage when it binds to HER2 receptors in lung tissue, Hurvitz noted. “[ILD] doesn’t seem to universally respond to steroids, so an immune-based underlying etiology is not entirely clear,” she said.
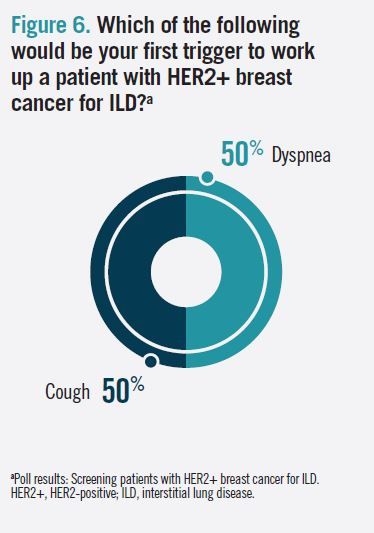
Audience poll respondents were evenly split on whether dyspnea (50%) or cough (50%) would be the first trigger to initiate a work-up for ILD (Figure 6). In a follow-up question about the typical initial response to grade 2 ILD in patients with HER2+ breast cancer, 50% responded “treatment discontinuation and steroids;” the remaining respondents were divided between “dose reduction and steroids” and “treatment hold and steroids.”
In the case patient, a CT performed 12 months after starting T-DM1 showed progression in the liver. She started on trastuzumab deruxtecan and had a response in the liver after 3 months, but a CT of the chest, abdomen, and pelvis showed an asymptomatic inflammatory pattern in the right lower lobe. The panelists generally agreed with erring on the side of caution regarding further treatment.
Iyengar said that he monitors patients very closely and discontinues the treatment immediately if a patient with mild symptoms shows any sign of worsening. “If it truly is ILD related to the drug, it can progress rapidly,” he said.
Hurvitz said that she stops therapy with grade 1 ILD and does not consider resuming therapy until imaging shows resolution.
Brufsky emphasized the importance of proactive management of patients with any sign of ILD. “I think that you’ve got to be very, very careful,” he said.
For patients with lung metastases, Hurvitz and Iyengar said that they would consider trastuzumab deruxtecan because data analysis from multiple studies did not find evidence that pulmonary metastases is a risk factor for ILD.
Iyengar added that caution should be exercised in patients with lymphangitic carcinomatosis but agreed that “you really want to try to get a response in the lungs, and using an active agent in this setting may be very helpful.”
Closing Remarks
At the conclusion of the discussion, Iyengar noted that the field of HER2+ breast cancer is a rapidly evolving space, with multiple clinical trials of therapies and combinations in different disease settings.
Hurvitz said she would like to see more data about managing leptomeningeal
metastases, which remains an unmet need.
Gadi predicted that the conversation about treatment of metastatic HER2+ breast cancer could be very different just 18 months from now. “I’m just really happy for our patients to have [so many] options available to them,” he said. “I don’t care so much about sequencing. At the end of the day, being able to get these drugs to those folks who need them when they need them…that’s probably the most important thing.”
References
- NCCN. NCCN Clinical Practice Guidelines in Oncology. Breast cancer, version 4.2021. Accessed May 7, 2021.
https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf - Hurvitz S, Vahdat L, Harbeck N, et al. HER2CLIMB-02: a randomized, double-blind, phase 3 study of tucatinib or placebo with T-DM1 for unresectable locally-advanced or metastatic HER2+ breast cancer [abstract]. Cancer Res. 2021;81(suppl 4).
doi:10.1158/1538-7445.SABCS20-OT-28-01 - Zimmer AS, Van Swearingen AED, Anders CK. HER2-positive breast cancer brain metastasis: a new and exciting landscape. Cancer Rep (Hoboken). 2020;e1274. doi:10.1002/cnr2.1274
- Awada A, Colomer R, Inoue K, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2(12):1557-1564. doi:10.1001/jamaoncol.2016.0237
- Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609. doi:10.1056/NEJMoa1914609
- Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610-621.
doi:10.1056/NEJMoa1914510 - Enhertu. Prescribing information. Daiichi Sankyo and AstraZeneca; 2021. Accessed May 10, 2021. https://dsi.com/prescribing-information-portlet/getPIContent?productName=Enhertu&inline=true
- Powell CA, Camidge DR, Gemma A, et al. Characterization, monitoring, and management of interstitial lung disease in patients with metastatic breast cancer: analysis of data available from multiple studies of DS-8201a, a HER2-targeted antibody drug conjugate with a topoisomerase I inhibitor payload. Presented at: 2018 San Antonio Breast Cancer Symposium. December 4-8, 2018. San Antonio, TX. Poster P6-17-06.
EP: 1.Patient Case: A 37-Year-Old Woman With HER2+/HR- Breast Cancer
EP: 2.Management Approaches for Patients with HER2+ BC at Risk for Brain Metastases
EP: 3.The Role of Prophylactic Therapy in HER2+ BC
EP: 4.Decision Making in Selecting Treatment in HER2+ BC
EP: 5.HER2+ BC: Considerations for Sequencing Treatment
EP: 6.Screening and Recognition of ILD in HER2+ BC
EP: 7.Monitoring Symptoms of ILD in HER2+ Breast Cancer
EP: 8.Management of ILD in HER2+ BC
EP: 9.The Evolving Space of HER2+ Breast Cancer
EP: 10.HER2-Positive Breast Cancer: Special Challenges and Expert Insight
Recap: Recent Advances in the Treatment of Metastatic Castration-Sensitive Prostate Cancer
September 18th 2022Expert oncologists review key studies in the metastatic castration-resistant prostate cancer treatment landscape and discuss how evidence can be applied to clinical practice to improve patient outcomes.
Recap: Updates in Treatment of HER2-Positive Breast Cancer and Brain Metastases
July 16th 2022Sara A. Hurvitz, MD; Stefania Maraka, MD; and Ruta Rao, MD, discuss the evolving landscape of metastatic HER2+ breast cancer, highlighting recent clinical trials and the management of patients with brain metastases.
Recap: Emory Experts Review Treatment Strategies for Transplant-Ineligible Multiple Myeloma
June 20th 2022A panel of experts from Emory University review several key data updates in multiple myeloma from recent meetings and discuss how the data can be applied to clinical practice to improve patient outcomes.
