Histologic Subtype in NSCLC: Does It Matter?
Since the publication of a meta-analysis in 1995 that demonstrated a modest survival benefit compared to best supportive care, platinum-based chemotherapy became the cornerstone of therapy in the first-line setting in advanced-stage non–small-cell lung cancer (NSCLC) for patients with good performance status.[1] A recent meta-analysis of 16 randomized trials including 2,714 patients demonstrated an advantage of chemotherapy over best supportive care with an absolute improvement in survival of 9% at 12 months.[2]
ABSTRACT: ABSTRACT:Platinum-based doublet chemotherapy remains the cornerstone of therapy in the first-line setting in advanced nonâsmall-cell lung cancer (NSCLC) for patients with good performance status. However, this paradigm has recently been challenged by the results of a study that showed a survival benefit with the addition of bevacizumab to carboplatin and paclitaxel in bevacizumab-eligible patients and by the superior efficacy of gefitinib and erlotinib compared to chemotherapy in epidermal growth factor receptor (EGFR) gene mutationâpositive tumors (mainly adenocarcinomas). In addition, histology has been recently recognized as a potential predictive factor in advanced NSCLC patients treated with chemotherapy. Prospective data from a preplanned subgroup analysis of a phase III study and retrospective reviews consistently reported a significant interaction between treatment by histology and response/survival in nonsquamous NSCLCs treated with pemetrexed, compared to squamous cell carcinoma (SCC). Thymidylate synthase, the main target of pemetrexed, was found to be differentially expressed among the histotypes of lung cancer, being lower in adenocarcinoma and higher in SCC and small-cell lung cancer. Thus, the availability of adequate amounts of tissue from biopsies to allow accurate pathologic subclassifications at diagnosis will be critical to help the oncologist select the most appropriate chemotherapy regimen as we move toward an individualized molecularly based approach.
Since the publication of a meta-analysis in 1995 that demonstrated a modest survival benefit compared to best supportive care, platinum-based chemotherapy became the cornerstone of therapy in the first-line setting in advanced-stage non–small-cell lung cancer (NSCLC) for patients with good performance status.[1] A recent meta-analysis of 16 randomized trials including 2,714 patients demonstrated an advantage of chemotherapy over best supportive care with an absolute improvement in survival of 9% at 12 months.[2]
Therapeutic guidelines indicate doublets of cisplatin or carboplatin with either gemcitabine (Gemzar), taxanes (paclitaxel or docetaxel [Taxotere]), or vinorelbine as reference regimens.[3,4] These guidelines are based on randomized phase III studies that compared these chemotherapy combinations “head to head” without detecting relevant differences in efficacy.[5-7] However, evidence from several randomized clinical trials and systematic reviews suggest that cytotoxic chemotherapy has reached an efficacy plateau.[8,9]
Currently the optimal therapeutic strategy for the individual patient diagnosed with stage IIIB or IV NSCLC is quite difficult to define since the decision-making process is shifting very quickly toward an individualized approach. Emerging evidence suggests that aside from the effects of well-known clinical parameters such as stage of disease and patient performance status, lung cancer behaves differently in different patients as the result of a variety of molecular profiles. The impact of histology in this context has been overlooked in the past.
The role of histology in addition to tumor-node-metastasis (TNM) stage was assessed in surgically managed stage I–IIIA NSCLC cases from the database of the International Association for the Study of Lung Cancer (IASLC), in the process of defining the new TNM staging system for lung cancer in the recently released 7th edition of the International Union Against Cancer and American Joint Committee on Cancer’s TNM Classification of Malignant Tumors. In 12,428 cases of NSCLC, age, gender, and performance status-but not histology-were found to be prognostic for survival after adjustment for stage of disease. Adenocarcinoma was more frequent than squamous cell carcinoma (SCC) in women (55% vs 25%), while the opposite was true in men (30% vs 57%). In a model including histology, pathologic stage, gender, and age, SCC patients had a significant survival advantage over those with adenocarcinoma or large-cell carcinoma (LCC), with no significant difference between LCC and adenocarcinoma. Cases designated as bronchioloalveolar carcinoma (BAC) had a superior prognosis compared with other histotypes. The better prognosis for SCC compared with other histotypes could not be demonstrated in women.[10]
In the same way, the Japanese Joint Committee of Lung Cancer Registry reported over 13,000 lung cancer cases that underwent surgery in 2002. They found a better prognosis for women and adenocarcinoma, with a 67% 5-year survival for adenocarcinoma vs 53% for SCC. In this study, the analysis of prognostic factors (including histology) was not adjusted for stage or gender; such adjustments could have led to different results.[11] In fact, by adjusting for stage and gender, any effect of imbalance that would favor adenocarcinoma is diminished, thus revealing a possible survival advantage for SCC.
Histology of Lung Cancer
Currently, the World Health Organization (WHO) classification of lung tumors should remain the common ground by which to compare results of studies potentially aimed at identifying a differential activity of any cytotoxic regimen according to histology.
In the past, oncologists dealing with lung cancer routinely distinguished only between small-cell lung cancer (SCLC) and NSCLC to select for the appropriate therapeutic management. NSCLC encompasses several subtypes with different morphologic features that are often treated according to similar strategies, so in everyday practice a clear-cut distinction among such histotypes was not considered mandatory.[12] Conversely, the WHO classifications of lung cancer have always kept the different histologic types separated, with individual categories for SCC, adenocarcinoma, and LCC.[13] Over the past few decades, the changing habits of cigarette smoking and enviromental pollution have contributed to changes in the relative frequency of these histologic subtypes. Adenocarcinoma shows a growing incidence, particularly in younger nonsmoking females, whereas SCC and SCLC are on the decline.[14]
The histologic recognition of classical SCC is generally straightforward, with distinctive areas of keratinization and an associated inflammatory component, especially in cases undergoing cavitation. Less differentiated forms of SCC, without keratinization and with smaller cells, may resemble basal cell layers of the squamous epithelium. In these cases, immunohistochemistry (IHC) may be required to detect specific types of cytokeratins, and neuroendocrine markers may also be helpful.
Adenocarcinoma includes a morphologically heterogeneous group of tumors that are less frequently associated with tobacco smoking than SCC. Adenocarcinomas are made of glands with papillary structures, or solid growth with mucin production.
BAC is still classified as an adenocarcinoma, although its pathologic definition has undergone major changes in recent years. Indeed, newly proposed strict criteria for the diagnosis of BAC are not correctly applied across all institutions, and when these criteria are followed, pure BAC cases become quite rare. The diagnosis of BAC is now restricted to tumors that have a lepidic growth along pulmonary septa in the absence of parenchymal invasion,[15] with further division into mucinous and nonmucinous forms. In clinical practice, however, the most common finding is adenocarcinoma showing a combined pattern of growth, with conventional acinar or papillary adenocarcinoma associated with mixed areas of BAC in the same tumor. Such tumors are classified as a mixed subtype of adenocarcinoma.
LCC is the least frequent variant of NSCLC, and it suffers from the lack of standardized morphologic diagnostic criteria. LCC underwent major changes in the last classification scheme, with undifferentiated pleomorphic and/or sarcomatoid variants moved to a new group designated as “sarcomatoid carcinoma.” The heading of LCC includes rarer lung cancer types, such as basaloid, lymphoepithelial, clear-cell, rhabdoid, and the large-cell neuroendocrine carcinoma (LCNEC). Most cases without clear signs of glandular or squamous differentiation are reported as large-cell (anaplastic) carcinoma . Most undifferentiated cases of LCC should probably be reclassified as adenocarcinoma or SCC on the basis of their molecular features. LCNEC should be identified using neuroendocrine markers and should be excluded from studies in NSCLC because of its different biologic and clinical properties.
Technical Problems Dealing With Limited Pathologic Specimens
A critical step is reached when considering the amount and the quality of the specimen that is received by the pathologist. In fact, diagnostic procedures routinely performed in thoracic oncology to obtain pathologic tissue tend to become increasingly less invasive. Nonetheless, cytology or small biopsies often yield a limited amount of viable cells or tissue, therefore allowing even an experienced pathologist to formulate a generic diagnosis of NSCLC–not otherwise specified (NOS).[16] In all these undifferentiated cases, IHC can assist pathologists in fine-tuning the diagnosis. Currently, markers such as thyroid transcription factor-1 (TTF-1), napsin and cytokeratin 7 (mainly for adenocarcinoma), and p63 and cytokeratin 5 (mainly for SCC) are more frequently used in the pathologic diagnostic process to trace signs of lineage differentiation.
Major advances in the phenotypic profiling of lung cancer were obtained by molecular analysis of a large number of genes, which provide different biologic signatures for each histologic subtype. As a result, a recent study described four genes that were upregulated in SCC by at least 20-fold in comparison to adenocarcinoma and/or normal lung parenchyma (PKP1, desmocollin-3 [DSC3], p63, and CK17).[17] The greatest difference between SCC and adenocarcinoma (and also between tumor and normal tissues) was observed for DSC3, a protein localized in desmosomal junctions of stratified epithelia. Gene-expression profiling data confirmed a differential expression of DSC3 in NSCLC.[18] DSC3 was expressed in almost half of a sample of undifferentiated LCC and was mutually exclusive with TTF-1, definining residual squamous differentiation of cells. Coexpression of the two markers is restricted to adenosquamous carcinomas. Thus, a combined morphologic-phenotypic approach may represent a valid diagnostic tool in lung cancer, as we move toward tailoring chemotherapy according to histology. Similarly, high-throughput microarrays have been used to measure microRNA expression levels in 122 adenocarcinoma and SCC samples and have identified hsa-miR-205 as a highly specific marker for SCC. Upon standardization, this diagnostic assay could provide highly accurate subclassification of NSCLC patients.[19]
The reproducibility of squamous vs nonsquamous classification could still largely be ameliorated. A study of 96 primary lung tumors showed that reproducible diagnosis of SCC based on morphology alone is inadequate. As expected in the era of histology-guided therapy, this highlights the need for stricter diagnostic criteria and confirmatory IHC stains in the diagnosis of SCC.[20]
Prognostic Impact of Histology
In NSCLC, tumor histology has not been consistently identified as a prognostic factor independent of treatment choice. Clinical studies evaluating the efficacy of chemotherapy in advanced NSCLC over a 25-year period have been reviewed, and a weak association between histology and therapeutic outcome has emerged, with a better median survival in patients with a nonsquamous histology. Indeed, none of the studies was planned to include a test of treatment-by-histology interaction. In 11 studies, an association between histology and prognosis was reported, while in 7 studies histologic subtype was even predictive of outcome after a specific chemotherapy regimen.
When evaluating trials of targeted therapies, 14 publications reported that histology was prognostic and/or predictive in patients treated with epidermal growth factor receptor (EGFR) tyrosine-kinase inhibitors (TKIs). A small study showed a nonsignificant trend to higher efficacy of cisplatin and etoposide vs a four-drug regimen in patients with SCC (response rate = 44% vs 21%, respectively) and not in patients with adenocarcinoma. Similarly, in a phase III study, nonsquamous histology was prognostic in patients treated with chemotherapy compared with best supportive care, while in patients with squamous histology no difference in outcome was seen. In a Greek study, patients with adenocarcinoma had a significantly greater response to gemcitabine plus docetaxel, whereas patients with SCC had a higher response rate to cisplatin plus docetaxel, but no differences were found in progression-free survival (PFS) and overall survival (OS).[21]
In an Italian phase III trial in advanced NSCLC testing three platinum-based doublets, no significant treatment-by-histology interaction was seen for survival. However, when pairwise comparisons of histology groups were analyzed, a statistically significant survival advantage in patients with SCC over adenocarcinoma became evident.
Recently, an individual patient data meta-analysis considered nine randomized clinical trials including almost 3,000 patients who were treated with doublets of cisplatin or carboplatin and a third-generation agent. Statistically significant tests of treatment-by-histology interaction confirmed the predictive effect of histology on survival. In nonsquamous cases, carboplatin-containing regimens were associated with lower odds of response and lower survival rates.[22]
These studies show how histology never really emerged as a strong prognostic factor that could predict response and/or survival. Hints of small differences suggesting a differential behavior of the tumor in relation to chemotherapy according to histology were there to be interpreted in later studies.
Differential Efficacy of Chemotherapy According to Histology
Until recently, only the coarse distinction between NSCLC and SCLC had relevant therapeutic implications. The doublet combination of platinum and etoposide is still unbeaten as the first-line approach in SCLC but is not used anymore in NSCLC. Historically, within the NSCLC subtypes there was no evidence of superior efficacy for a specific cytotoxic drug administered as a single agent or as part of a combination. Based on the results of randomized clinical trials, each institution or medical oncologist has been choosing a doublet, mainly based on local policies, costs, or convenience of the therapeutic schedule.
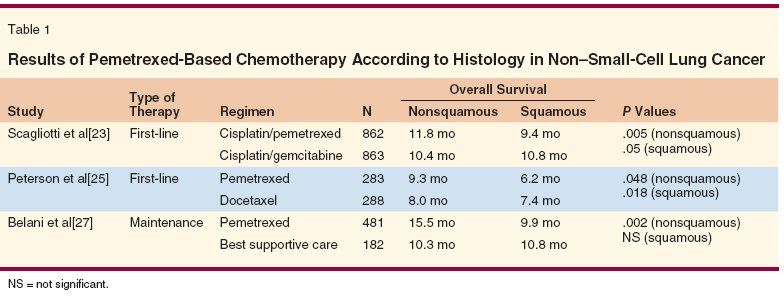
Recently, however, new findings prompted a change in clinical practice. The therapeutic advantage of pemetrexed (Alimta) and cisplatin compared to gemcitabine and cisplatin in nonsquamous NSCLC[23] and the safety issues regarding the use of bevacizumab (Avastin) in squamous NSCLC[24] brought up histology as a new evidence-based selection criterion for choosing the most appropriate chemotherapy regimen. The first study was a phase III multicenter trial including 1,725 chemotherapy-naive patients with advanced NSCLC who were randomized to receive cisplatin and pemetrexed or cisplatin and gemcitabine (Table 1). The trial was planned according to a noninferiority study design with OS as a primary endpoint. Preplanned subset analyses were carried out to evaluate differential efficacy according to histology. Patients with adenocarcinoma (847 patients) and with LCC (153 patients) had a longer OS (12.6 vs 10.9 months and 10.4 vs 6.7 months, respectively) when they were treated with cisplatin and pemetrexed. Conversely, the 473 patients with SCC had a survival advantage when they received cisplatin and gemcitabine (10.8 vs 9.4 months). A significant treatment-by-histology interaction was observed for both PFS and OS. As a result, pemetrexed received worldwide approval for use in combination with cisplatin for first-line treatment in patients with locally advanced or metastatic nonsquamous NSCLC.
The same trend was seen in a retrospective analysis from a second-line study of pemetrexed vs docetaxel.[25] Patients with nonsquamous histology treated with pemetrexed had a statistically significant superior survival compared to those treated with docetaxel (hazard ratio [HR] = 0.778, 95% confidence interval [CI] = 0.607–0.997), whereas in patients with SCC, docetaxel was superior to pemetrexed (HR = 1.563, 95% CI = 1.079–2.264). The investigators found a significant treatment-by-histology interaction. In addition, a phase II randomized study conducted in Japan compared two doses (500 vs 900 mg) of single-agent pemetrexed as second- or third-line therapy in advanced NSCLC and also showed that efficacy varied according to histology.[26]
Pemetrexed was also tested in a phase III study in comparison to placebo as maintenance therapy in advanced NSCLC that had not progressed after four cycles of front-line platinum-based standard chemotherapy. PFS was the primary endpoint of the study and was significantly longer with pemetrexed (4 vs 2 months).
Overall survival was also significantly better in the pemetrexed arm (13.4 vs 10.6 months). The improvements in PFS and OS were more striking in adenocarcinoma (PFS = 4.4 vs 1.8 months, HR = 0.47; OS = 15.5 vs 10.3 months; HR = 0.70), while pemetrexed did not improve either outcome parameter in SCC.[27]
The bulk of data coming from the three above mentioned studies supports a higher efficacy of pemetrexed in adenocarcinoma, either as first-line or second-line therapy or as a maintenance strategy. A potential explanation for this biologic phenomenon relies in the mechanism of action of pemetrexed. Thymidylate synthase (TS) is the main cellular target of pemetrexed, and higher levels of TS promote resistance to pemetrexed action, as seen in a variety of solid tumors.[28] In lung cancer, a differential expression of TS among histotypes has been extensively described. Baseline TS gene and protein expressions are significantly higher in SCC compared to adenocarcinoma.[29] In addition, TS and S phase kinase–associated protein (Skp2) are transcriptionally regulated in the S phase of the cell cycle by the transcription factor E2F1, and elevated expression of Skp2 has been found more commonly in patients with SCC than in those with adenocarcinoma.[30]
In LCC, significantly higher median TS levels were found compared to adenocarcinoma, but no correlation was found between TS and either Ki-67 or E2F1. Also in LCC, significantly higher TS levels were observed in DSC3-positive tumors (resembling SCC differentiation) compared to DSC3-negative tumors, and lower TS levels were seen in TTF-1 positive LCC (resembling adenocarcinoma differentiation).[31] Finally, the highest levels of TS gene and protein expression were registered in SCLC.[32] A recent phase III study in extensive-disease SCLC, which investigated the combination of pemetrexed and carboplatin vs etoposide and carboplatin, was stopped early due to the lack of efficacy seen in the pemetrexed arm.[33]
Histology and Targeted Therapies
Advances in the understanding of the molecular biology of cancer have made possible the discovery of several molecular targets and the consequent development of novel therapies with the primary goal of switching off signaling pathways involved in the uncontrolled growth of cancer cells. Inhibition of EGFR signaling and blockade of angiogenesis have been identified as key therapeutic targets in NSCLC. Strategies to block these pathways mainly include TKIs and monoclonal antibodies.
Erlotinib (Tarceva) and gefitinib (Iressa) are the two EGFR-TKIs with proven efficacy against NSCLC. Recent phase II studies have shown that both agents could be more active than chemotherapy as first-line regimens in advanced NSCLC when the population is selected for the presence of EGFR mutations or EGFR gene amplification,[34] as detected by fluorescence in situ hybridization (FISH). In 2009, two papers reported about the use of TKIs as first-line therapy in selected patients with advanced NSCLC. Both studies showed significant improvements in response rate and PFS in patients whose tumors had EGFR mutations. A Spanish group screened over 2,000 patients for the presence of EGFR mutations, which were found in 16% of cases and, as expected, were more frequent in women, adenocarcinomas, and nonsmoking patients.[35] Patients who presented with an EGFR mutation were treated with upfront erlotinib, and had a median survival of 27 months.
In the Iressa Pan-Asia Study (IPASS), gefitinib was compared to carboplatin/paclitaxel as first-line treatment in 1,217 never (or light) smokers with Asian ethnicity and a histologic diagnosis of adenocarcinoma.[36] This study demonstrated the superiority of gefitinib relative to the carboplatin/paclitaxel doublet in terms of PFS (12-month PFS = 25% vs 7%). EGFR gene mutations were assessed in 36% of all randomized patients. The benefit was largely confined to patients harboring that mutation, and it may not depend strictly on histology. Overall survival was not prolonged by gefitinib, and this remains a key issue to be addressed by future trials.
New results from the phase III Sequential Tarceva in Unresectable NSCLC (SATURN) trial presented at the 13th World Conference on Lung Cancer, organized by the IASLC, show that erlotinib maintenance therapy after first-line platinum-based doublets in almost 889 patients with advanced nonprogressing NSCLC significantly improves OS (12 vs 11 months).[37] Survival was a secondary endpoint of this trial. PFS, the primary endpoint, was increased 41% to 45% by erlotinib. Subgroup analyses for clinical predictive factors did not show any difference in outcome. Unexpectedly, all patients-regardless of sex, histology, and nonsmoking status-derived some benefit from the drug. The investigators found a 10% absolute difference in survival between the treatment and placebo groups at 3 years. However, the most consistent improvement of outcome parameters was recorded in the small subset of patients with EGFR mutation.
Clinical parameters may help the clinician to foresee sensitivity to EGFR TKIs. Female sex, Asian ethnicity, nonsmoker status, and adenocarcinomatous histology predicted for the presence of EGFR mutations and, therefore, can be considered clinical predictors of response.[38] In the end, EGFR mutations are mostly present in adenocarcinoma rather than SCC, but the future wider availability of kits to test for EGFR mutations will overcome the need to choose EGFR TKIs vs cytotoxic chemotherapy based solely on histotype. Conversely, such clinical parameters did not apply to the monoclonal antibody cetuximab (Erbitux) in the First-Line Erbitux in Lung Cancer (FLEX) trial, where cetuximab was added to the combination of cisplatin and vinorelbine and prolonged survival compared to chemotherapy alone.[39] Data do not support the hypothesis that KRAS mutation status is predictive for cetuximab efficacy when combined with chemotherapy, as seen previously in colon carcinoma.[40] Only the emergence of early cutaneous rash was predictive for outcome.[41] Patients treated with cetuximab who developed an early acne-like rash of any grade had a longer median OS than those without the rash.
A new molecular subset of NSCLC is defined by the expression of EML4-ALK. Patients most likely to harbor the EML4-ALK fusion oncogene are young, mainly male, never/light smokers with adenocarcinoma. Because some of these features are also associated with EGFR mutation, it is essential to screen such patients by mutation testing and not to rely solely on the presence of clinical predictors. EML4-ALK expression is mutually exclusive to EGFR and KRAS mutations.[42] EML4-ALK allows a step further in defining newer subsets of adenocarcinoma based on genomic profiling. They represent probably around 5% of NSCLC patients. Those who harbor this mutation do not benefit from EGFR TKIs and should be directed to trials with ALK-targeted inhibitors.
Coming back to the other disrupted pathway in lung cancer, it is well recognized that neoangiogenesis, the formation of new blood vessels, is a fundamental process for the growth of solid tumors and their metastatic spread. Bevacizumab is a recombinant, humanized, monoclonal antibody against vascular endothelial growth factor (VEGF), and is currently the most extensively investigated antiangiogenic agent.
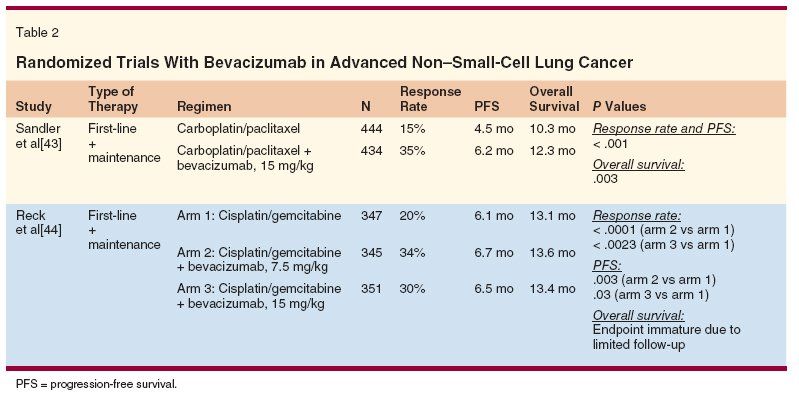
In a phase II study of bevacizumab and chemotherapy, fatal hemorrhagic episodes turned out to be more frequent when central cavitated lesions were present, as is more frequently observed in SCC.[24] Therefore, squamous histology, metastases to the central nervous system, history of hemoptysis, and history of documented hemorrhagic diathesis became exclusion criteria for phase III studies. Once again, histotype is a potential limitation in the use of a new drug, with bevacizumab use favored for adenocarcinoma.
In a phase III trial (Eastern Cooperative Oncology Group [ECOG] 4599), the combination of bevacizumab and paclitaxel/carboplatin followed by bevacizumab until disease progression showed an improvement in overall response rate (35% vs 15%), PFS (6.2 vs 4.5 months), and OS (12.3 vs 10.3 months) over paclitaxel/carboplatin alone (Table 2).[43] A pan-European study following a similar design but with cisplatin/gemcitabine as the chemotherapy backbone met the primary endpoint of improving PFS but did not confirm any survival gain,[44] probably due to poststudy therapies administered when disease progressed. In both studies, greater toxicity was seen for patients receiving bevacizumab, especially in terms of hypertension, proteinuria, bleeding, neutropenia, and thrombocytopenia. A recent multicenter phase II study of pemetrexed and carboplatin plus bevacizumab followed by maintenance pemetrexed and bevacizumab demonstrated encouraging activity in patients with chemotherapy-naive advanced nonsquamous NSCLC (response rate of 55% and OS of 14 months).[45]
A new class of targeted agents is represented by inhibitors of insulin-like growth factor type 1 receptor (IGF-IR). IGF-IR is often overexpressed in lung tumors and mediates the proliferation of lung cancer cells and their resistance to therapy. It has been reported to be expressed at higher levels in SCC than in adenocarcinoma. The IGF-IIR gene is mutated in approximately 60% of SCC cases and 27% of adenocarcinomas.[46] Therefore, a promising strategy for inhibiting the function of IGF-IR is the use of antibodies that bind to the extracellular domain of this receptor.
CP-751,871 (figitumumab) is a fully human monoclonal antibody that proved active in NSCLC when associated with carboplatin/paclitaxel treatment in a phase II study conducted in chemotherapy-naive patients.[47] A tendency toward higher response rates (70%) was observed in SCC compared to adenocarcinoma with the addition of CP-751,871 to carboplatin and paclitaxel, whereas NSCLC-NOS patients (almost one-third of those studied) did not receive any additional benefit. Such data reflect a differential expression of IGF-IR across NSCLC subtypes and, once more, highlight the need for a more precise histologic definition.
Conclusions
NSCLC results from a multistep process linked to several intracellular pathways and as a sum of multiple genetic alterations. Data from available randomized studies have yielded sufficient evidence that chemosensitivity varies consistently according to histotype. Even within a morphologically defined histologic subtype such as adenocarcinoma, multiple subtypes can exist, each associated with a different prognosis and/or responsiveness to a specific drug. In a subset of adenocarcinoma of the lung, positivity for alpha and beta estrogen receptor expression was associated with distinctive clinicopathologic and genetic features. Alpha estrogen receptor expression correlated with EGFR mutations and represented a worse prognostic factor.[48]
Future research should be aimed at exploiting combinations of drugs to target different pathways at the same time, while implementing a central pathology review (or other related methods) to verify histologic diagnosis and improve trial designs based on histology.
References:
1. Non-small Cell Lung Cancer Collaborative Group: Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 311:899-909, 1995.
2. NSCLC Meta-Analyses Collaborative Group: Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: A systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 26:4617-4625, 2008.
3. Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol 15:2996-3018, 1997.
4. D’Addario G, Felip E, for the ESMO Guidelines Working Group: Non-small-cell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20(suppl 4):68-70, 2009.
5. Scagliotti GV, De Marinis F, Rinaldi M, et al: Phase III randomized trial comparing three-platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 20:4285-4291, 2002.
6. Kelly K, Crowley J, Bunn PA Jr, et al: Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced nonâsmall-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol 19:3210-3218, 2001.
7. Fossella F, Pereira JR, von Pawel J, et al: Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. J Clin Oncol 21:3016-3024, 2003.
8. Delbaldo C, Michiels S, Rolland E, et al: Second or third additional chemotherapy drug for non-small cell lung cancer in patients with advanced disease. Cochrane Database Syst Rev (4):CD004569, 2007.
9. Breathnach OS, Freidlin B, Conley B, et al: Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: Sobering results. J Clin Oncol 19:1734-1742, 2001.
10. Sculier J-P, Chansky K, Crowley J, et al: The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol 3:457-466, 2008.
11. Asamura H, Goya T, Koshiishi Y, et al: Japanese Joint Committee of Lung Cancer Registry. A Japanese lung cancer registry study. Prognosis of 13,010 resected lung cancers. J Thorac Oncol 3:46-52, 2008.
12. Hirsch FR, Speafico A, Novello S, et al: The prognostic and predictive role of histology in advanced non-small cell lung cancer: A literature review. J Thorac Oncol 3:1468-1481, 2008.
13. Travis WD, Brambilla E, Muller-Hermelink HK, et al: Pathology and genetics of tumors of the lung, pleura, thymus and heart, in WHO Classification of Tumours, pp 9-124. IARC Press, Lyon, 2004.
14. Alberg AJ, Ford JG, Samet JM: Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines, 2nd ed. Chest 132:29S-55S, 2007.
15. Travis WD, Garg K, Franklin WA, et al: Evolving concepts in the pathology and computed tomography imaging of lung adenocarcinoma and bronchioloalveolar carcinoma. J Clin Oncol 23:3279-3287, 2005.
16. Cataluna JJ, Perpina M, Greses JV, et al: Cell type accuracy of bronchialbiopsy specimens in primary lung cancer. Chest 109:1199-1203, 1996.
17. Angulo B, Suarez-Gauthier A, Lopez-Rios F, et al: Expression signatures in lung cancer reveal a profile for EGFR-mutant tumours and identify selective PIK3CA overexpression by gene amplification. J Pathol 214:347-356, 2008.
18. Monica V, Ceppi P, Righi L, et al: Further subtyping of undifferentiated large cell carcinoma of the lung at the advent of histotype-specific chemotherapy. Modern Pathol 22:709-717, 2009.
19. Lebanony D, Benjamin H, Gilad S, et al: Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J Clin Oncol 27:2030-2037, 2009.
20. Grilley-Olson JE, Hayes DN, Qaqish BF, et al: Diagnostic reproducibility of squamous cell carcinoma (SC) in the era of histology-directed non-small cell lung cancer (NSCLC) chemotherapy: A large prospective study (abstract 8008). J Clin Oncol 27(15S):409s, 2009.
21. Georgoulias V, Papadakis E, Alexopoulos A, et al: Platinum-based and non-platinum-based chemotherapy in advanced non-small-cell lung cancer: A randomised multicentre trial. Lancet 357:1478-1484, 2001.
22. Ardizzoni A, Boni L, Tiseo M, et al, for the CISCA (CISplatin versus CArboplatin) Meta-analysis Group: Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: An individual patient data meta-analysis. J Natl Cancer Inst 99:847-857, 2007.
23. Scagliotti GV, Parikh P, von Pawel J, et al: Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543-3551, 2008.
24. Johnson DH, Fehrenbacher L, Novotny WF, et al: Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22:2184-2191, 2004.
25. Peterson P, Park K, Fossella F, et al: Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC). J Thorac Oncol 2:S851, 2007.
26. Okabe T, Kubota K, Tamura T, et al: Prognostic factors affecting survival on pretreated patients with locally advanced or metastatic non-small cell lung cancer (NSCLC)-subgroup analysis in a randomized Ph II study of pemetrexed 500 mg/m2 and 1000 mg/m2. Eur J Cancer Suppl 5(4):376, 2007.
27. Belani CP, Brodowicz Y, Ciuleanu T , et al: Maintenance pemetrexed (Pem) plus best supportive care (BSC) versus placebo (Plac) plus BSC: A randomized phase III study in advanced non-small cell lung cancer (NSCLC) (abstract CRA8000). J Clin Oncol 27(18S), 2009.
28. Shih C, Chen VJ, Gossett LS, et al: LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res 57:1116-1123, 1997.
29. Ceppi P, Volante M, Saviozzi S, et al: Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 107:1589-1596, 2006.
30. Salon C, Merdzhanova G, Brambilla C, et al: E2F-1, Skp2 and cyclin E oncoproteins are upregulated and directly correlated in high-grade neuroendocrine lung tumors. Oncogene 26:6927-6936, 2007.
31. Scagliotti GV, Monica V, Ceppi P, et al: Baseline thymidylate synthase expression according to histological subtypes of non-small cell lung cancer (abstract 7521). J Clin Oncol 27(15S):387s, 2009.
32. Ceppi P, Volante M, Ferrero A, et al: Thymidylate synthase expression in gastroenteropancreatic and pulmonary neuroendocrine tumors. Clin Cancer Res 14:1059-1064, 2008.
33. Smit F, Socinski MA, Mullaney BP, et al: Pharmacogenomic analysis from a phase III study of pemetrexed plus carboplatin (PC) versus etoposide plus carboplatin (EC) in chemonaive patients (pts) with extensive-stage disease small cell lung cancer (ED-SCLC) (abstract 8030). J Clin Oncol 27(15S):414s, 2009.
34. Hirsch FR, Varella-Garcia M, Bunn PA Jr, et al: Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 24:5034-5042, 2006.
35. Rosell R, Moran T, Queralt C, et al: Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361:958-967, 2009.
36. Mok TS, Wu Y-L, Thongprasert S, et al: Gefitinib or carboplatinâpaclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947-957, 2009.
37. Cappuzzo F, Coudert B, Wierzbicki R, et al: Efficacy and safety of erlotinib as first-line maintenance in NSCLC following non-progression with chemotherapy: Results from the phase III SATURN study (abstract A2.1). J Thorac Oncol 4(S1), 2009.
38. Sequist LV, Bell DW, Lynch TJ, et al: Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol 25:587-595, 2007.
39. Pirker R, Pereira JR, Szczesna A, et al: Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet 373:1525-1531, 2009.
40. Mack PC, Holland WS, Redman M, et al: KRAS mutation analysis in cetuximab-treated advanced stage non-small cell lung cancer (NSCLC): SWOG experience with S0342 and S0536 (abstract 8022). J Clin Oncol 27(15S):412s, 2009.
41. O’Byrne KJ, Bondarenko I, Barrios C, et al: Molecular and clinical predictors of outcome for cetuximab in non-small cell lung cancer (NSCLC): Data from the FLEX study (abstract 8007). J Clin Oncol 27(15S):408s, 2009.
42. Shaw AT, Yeap BY, Mino-Kenudson M, et al: Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27:4247-4253, 2009.
43. Sandler A, Gray R, Perry MC, et al: Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542-2550, 2006.
44. Reck M, von Pawel J, Zatloukal P, et al: Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27:1227-1234, 2009.
45. Patel JD, Hensing TA, Rademaker A, et al: Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol 27:3284-3289, 2009.
46. Pavelic J, Krizanac S, Kapitanovic S, et al: The consequences of insulin-like growth factors/receptors dysfunction in lung cancer. Am J Respir Cell Mol Biol 32:65-71, 2005.
47. Karpp DD, Paz-Ares LG, Novello S, et al: Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol 27:2516-2522, 2009.
48. Raso MG, Behrens C, Herynk MH, et al: Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res 15:5359-5368, 2009.