HPV and the Immune System in Head and Neck Cancers: Therapeutic Considerations
A review of the role of immune therapy in HPV-associated head and neck squamous cell carcinoma, along with the evidence and perspective behind differing therapeutic considerations.
Dr Ganti is a professor of internal medicine and biochemistry at the University of Nebraska Medical Center and a staff physician at the VA Nebraska Western Iowa Health Care System in Omaha, NE. His laboratory is focused on evaluating new pathways in head and neck and lung cancer and identifying novel prognostic and predictive biomarkers for these malignancies.

Ms Grinnell is a third-year medical student at the University of Nebraska Medical Center in Omaha, NE. She is completing an enhanced medical education Track in Immunology and is active in research with a particular interest in immunotherapy and autoimmunity.

Dr Krishnan is a second year of fellow in hematology-oncology at University of Nebraska Medical Center. Her research interests are immunotherapy and novel therapies for solid tumor malignancies.

TABLE 1. PD-1-Targeted Immunotherapies for HPV-associated HNSCC
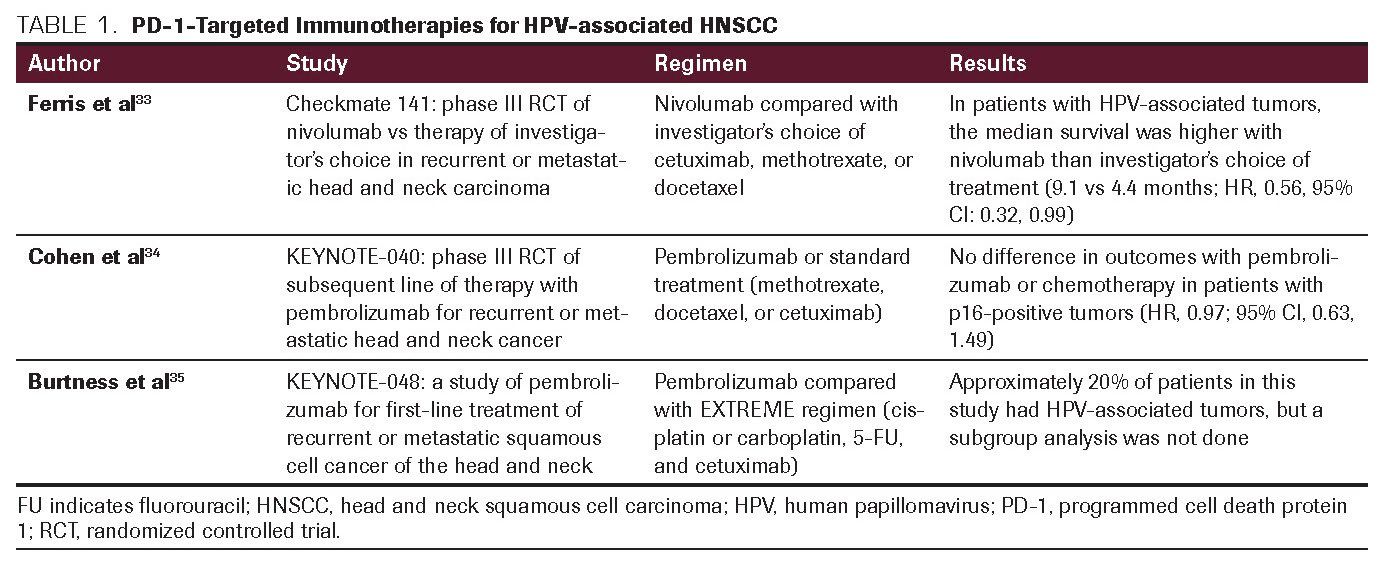
TABLE 2. PD-1-Targeted Immunotherapies Currently Under Investigation in the Treatment of HPV-Associated HNSCC
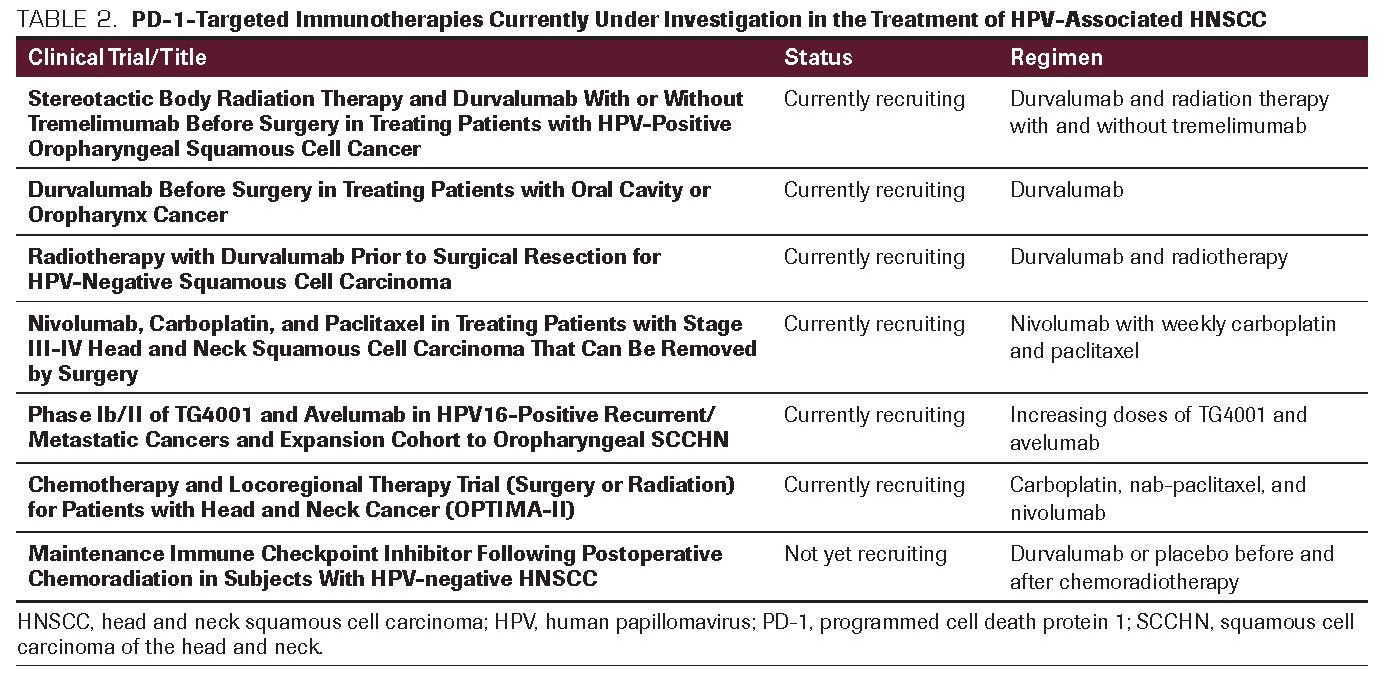
TABLE 3. Biomarkers Being Investigated to Predict PD-1/PD-L1 Inhibitor Response
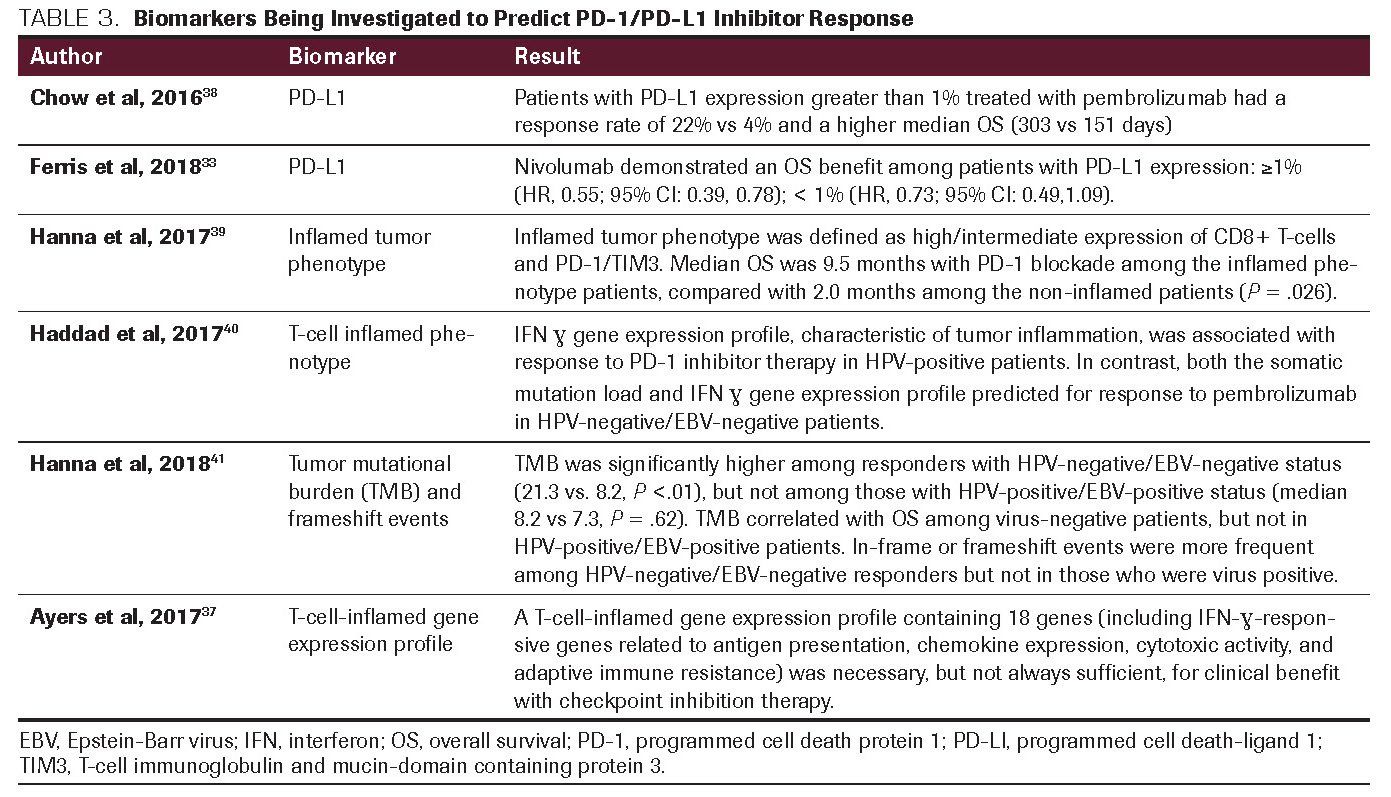
TABLE 4. Other Immunotherapies Under Investigation for the Treatment of HPV-Associated HNSCC
Immunotherapy applications for head and neck squamous cell carcinoma (HNSCC) are rapidly evolving. The progress towards immunotherapy has demonstrated improved outcomes for patients with HNSCC. Human papillomavirus (HPV)-associated HNSCC has a much better prognosis and differs from HPV-negative HNSCC in its genomic and immunologic profile, with strikingly higher immune cell activation and infiltration. Despite an increased incidence of HPV-associated HNSCC, and differences in immune signature based on HPV status, the management does not differ from non-HPV tumors. Clinical trials are ongoing to integrate immunotherapy in the management of early- and late-stage HNSCC, and its current use is limited to the metastatic setting. This article describes the role of immune therapy in HPV-associated HNSCC along with the evidence and perspective behind differing therapeutic considerations.
Introduction
In the United States, head and neck squamous cell carcinoma (HNSCC) accounts for 4% of all malignancies; its estimated annual incidence is 53,260, and HNSCC was responsible for 10,750 deaths in 2020. 1 In addition to the traditional risk factors of alcohol and tobacco use, human papillomavirus (HPV) has been implicated in the pathogenesis of HNSCC. The incidence of HPV-positive oropharyngeal cancer has been increasing in the United States and worldwide. Although HPV positivity is seen in all areas of the head and neck, the majority of evidence on the effect of HPV in cancer biology and prognosis is in the oropharyngeal cancers; estimates from the American Cancer Society suggest that pharyngeal cancers make up about 17,950 new cases and were attributed to 3640 deaths in 2020.1 The treatment implications of HPV-associated HNSCC are important, as the risk of death has been shown to be 60% to 80% lower with conventional therapy as compared with HPV-negative oropharyngeal cancers.2,3
A study published in 2006 by Weinberger et al found that molecular classification suggested a favorable prognosis in a subset of patients with HPV-associated oropharyngeal cancers that overexpressed p16 with lower levels of p53 and Rb expression.4 The DAHANCA 5 (Danish Head and Neck Cancer Group 5) trial demonstrated that p16 positivity was associated with a 5-year locoregional control rate of 58% and an overall survival (OS) of 62%.5 Although conventional treatments including surgery, radiation, and conventional chemotherapy have good efficacy, patients who relapse with distant metastasis often have dismal outcomes.6,7 These poor outcomes following relapse after conventional treatment have led to a call for more research into tumor-specific targeted therapy. Immunotherapies offer promising treatment potential as tumors in the head and neck generally infiltrate important structures and complete surgical resection may be difficult, leading to a poor overall prognosis.8
Pathogenesis of HPV
HPV is a virus that can infect the skin and mucosal surface of the oral pharynx, throat, and anogenital region.9 HPV infection is common, and it is thought to occur through mucosal contact with an infected sexual partner. Patients with HPV-associated HNSCC are usually younger and healthier, with less lifestyle risk factors than patients who do not have HPV-driven tumors.10 A key aspect of HPV infection and the development of HPV-positive HNSCC is that it occurs in the setting of a persistent HPV infection.
Evolution of cancer is a multistep process whereby cells acquire a number of traits that enables transition from normal to neoplastic behavior. Ineffective immune response results in what may be a protracted state of inflammation. While the heightened immune response should be associated with robust antitumoral activity, the inflammatory response has the paradoxical effect of promoting tumorigenesis. Further intriguing is that the tumor-enhancing effect is disproportionately mediated by cells of the innate immune system.
A recent study conducted by Sonawane et al found that 1 in 9 men between the ages of 18 to 69 was infected with oral HPV.11 Several strains of HPV cause infection, some benign and some with high-risk potential. DNA from high-risk HPV strains may be integrated into the genome of the human cell, which can lead to an overexpression of viral oncogenes E6 and E7. Viral oncogene E6 is thought to bind cycle cell–regulatory protein p53, leading to its degradation and subsequent uncontrolled tumor cell growth. E7 has an additive oncogenic function whereby it binds to and degrades Rb, a tumor suppressor that blocks exit from the G1 phase of the cell cycle, thus regulating E2F transcription factors.12
HPV infection is now responsible for the largest number of oropharyngeal tumors in the United States, accounting for 45% to 90% of cases.13,14 HPV positivity is detected in 25% of all HNSCCs.15 Notably, survival is markedly higher in patients with HPV-associated HNSCC compared with those with HPV-negative HNSCC (3-year survival rate of 84% compared with 57%, respectively).16
Immune Landscape of HPV-Associated HNSCC
Research has identified that HPV-positive head and neck cancers are a distinct subset, genetically and molecularly.17 There are several mechanisms by which immune evasion in these tumors may be explained. Some of these include interference with antigen presentation, modulation of transcriptional activity of genes, upregulation of cytokines, and interaction of immune checkpoint receptor signaling. HPV-positive HNSCCs have more markers of immune infiltration and CD8+ T-cell activation than HPV-negative HNSCCs, a potential target for harnessing adaptive antitumor immune response systems.18 Infection with high-risk HPV types (HPV 16, 18, 31, and 33) plays a causal role in the pathogenesis of oropharynx squamous cell carcinoma (OPSCC), and has distinct clinical and molecular features. More than 90% of HPV-associated OPSCC in the United States is attributed to HPV high-risk type 16, with rare accounts of HPV type 18, 33, and others reported as responsible in the literature.19
In addition to higher levels of CD8+ T-cell activation, HPV-positive tumors also have higher expression of perforin and granzyme A and B, indicating immune activity.20, 21 Many mechanisms of cell cycle regulation escape have been described in HPV-positive HNSCC, including the development of T-cell tolerance to HPV infection, reduced regulation by interferon gamma, unbalanced signal transducer and activator of transcription (STAT) 1 and 3 signaling, secretion of immunosuppressive cytokines, expression of programmed cell death-ligand 1 (PD-L1), and downregulation of human leukocyte antigen expression and processing which allows the immune system to recognize and tag tumors before destruction.22 Higher expression of the immunoinhibitory receptor cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) has been found in HPV-positive HNSCC (P = .003) and appears to have a correlation with higher regulatory T cells (Tregs) expression (r = 0.866, P <.00001).17
CTLA-4 is a protein receptor that like PD-L1, works to downregulate the immune system’s natural response to attack tumor cells and is generally expressed on Tregs. Interferon gamma (IFN-É£) has also shown to play a role in HPV-positive HNSCC pathogenesis. Escape from detection by cytotoxic T cells is thought to be related to IFN-É£ deficiency, which activates STAT1 transcription to prevent tumor escape from regulation.23, 24
Current research surrounds treatment that may alter the tumor’s inflammatory and pro-growth environment, including toll-like receptor stimulation, inhibition of interleukin-6 (IL-6) pathways, immune checkpoint inhibition, and modulation of Tregs and macrophages.25 Viral oncogenes E6 and E7 are potential targets for immune intervention, as are T cells that express programmed cell death protein 1 (PD-1), a marker that has a self-tolerance-promoting and negative regulatory role on T cells, which typically attenuates infective processes and tumor proliferation.26
Existing Literature on the Role of Immunotherapy in HPV-Associated HNSCC
Currently, treatment of HPV-associated oropharyngeal carcinoma resembles non-HPV oral cancers. Although clinical trials evaluating de-escalation of therapy are underway, HPV status does not yet alter the standard treatment course. The current management of oropharyngeal carcinoma utilizes a combination of chemotherapy, radiation, and possibly surgery. However, if patients develop metastatic disease, options are mainly systemic treatments with a palliative intent. There is increasing consensus that HPV-associated HNSCC may benefit from less intensive management regimens; however, presently only retrospective data support this. There are several ongoing clinical trials analyzing de-intensified regimens for HPV-positive oropharyngeal cancer. Overall, the strategies include modification of systemic therapies, surgical procedures, radiation dosing and techniques, and use of immunotherapy or targeted therapies.
Significant improvements in survival have been observed with PD-1 inhibitors in melanoma, non–small cell lung cancer, and renal cell carcinoma.27-29 PD-1 and PD-L1 are proteins present on the surface of cells that function to distract the immune system from attacking tumor cells.30 HPV-positive tumors have increased expression of PD-L1, making PD-1 inhibitors a promising therapeutic option.31 Promising clinical trial results led the FDA approval of pembrolizumab for treating refractory or metastatic HNSCC in 2016.32 Patients enrolled in the nonrandomized KEYNOTE-012 trial had metastatic HNSCC, which had progressed despite treatment with standard therapies. A total of 16% of patients experienced a response to pembrolizumab. In 82% of these patients, the response lasted for more than 6 months and for several, the response lasted for more than 2 years.32
In the phase III CheckMate 141 study, nivolumab treatment resulted in longer overall survival (OS) as a single therapy agent compared with a standard treatment (median OS of 7.5 months compared with 5.1 months, respectively), with estimates of 1-year survival 19% higher with nivolumab.33 For patients with p16-positive status, the median survival in Checkmate 141 was 9.1 months with nivolumab compared with 4.4 months with standard treatment (HR, 0.56; 95% CI: 0.32, 0.99).33 The KEYNOTE-040 trial randomized patients to pembrolizumab or standard of care chemotherapy for metastatic HNSCC. The median OS in the pembrolizumab group was 8.4 months compared with 6.9 months in the standard treatment group.34 However, in contrast to the results of the CheckMate 141 study, pembrolizumab was not superior to chemotherapy in patients with p16-positive disease (HR, 0.97; 95% CI: 0.63, 1.49).
The KEYNOTE-048 trial compared survival and disease progression in previously untreated patients with recurrent or metastatic HNSCC given pembrolizumab monotherapy or pembrolizumab with chemotherapy (EXTREME regimen: platinum, 5-fluorouracil and cetuximab).35 Data presented recently showed that pembrolizumab monotherapy improved OS by 39% (P = .0007) in patients whose tumors expressed PD-L1 (combined positive score ≥20) compared with chemotherapy. The median OS was 12.3 months versus 10.3 months, respectively. The benefit was higher in patients with a CPS of at least 20, where the median OS was 14.9 compared with 10.7 months (HR, 0.78; 95% CI: 0.64, 0.96; P = .008). When pembrolizumab alone was compared with chemotherapy plus cetuximab, the median OS in the total population favored the combination (13 vs 10.7 months; HR, 0.77; 95% CI: 0.63, 0.93; P = .0034).35 While approximately 20% of patients in this study were p16-positive, they were not analyzed separately. These studies have been summarized in Table 1. Many trials assessing combination regimens with PD-1 inhibitors and HPV vaccine studies are currently recruiting patients. These studies are outlined in Table 2.
Prediction of Response to Immune Checkpoint Inhibitors
While tumor mutational burden (TMB) has been investigated as a biomarker to study sensitivity to checkpoint inhibition in different malignancies, PD-L1 expression does not always predict who will have an increased response. More studies are needed, potentially utilizing both TMB and PD-L1 assays, to predict response.36 Some of the results assessing biomarkers to predict response to checkpoint inhibitors are shown in Table 3.
Currently, several other markers in addition to PD-L1 expression have been identified and are being evaluated. These include HPV expression, IFN-É£ signature score, microsatellite instability, and neoantigen production.42 IFN-É£ related mRNA gene expression signatures have predicted clinical response to pembrolizumab in melanoma with some indication that IFN-É£ and T cell-associated inflammatory genes may predict PD-1 blockade response.37 However, none of these biomarkers have consistently shown to be predictive for response to immune checkpoint inhibitors and further studies are needed.
Other Immunotherapies in HNSCC
In addition to PD-L1 and CTLA-4 inhibitors, other monoclonal antibodies are being investigated in HPV-positive HNSCC treatment including monalizumab, a monoclonal antibody targeting natural killer group 2 member A (NKG2A). The NKG2A heterodimeric receptor is one of the most prominent NK inhibitory receptors. Monalizumab blocks cancer cells from evading immune system detection by facilitating activation of natural killer and cytotoxic T cells. 43 IL-6 is another target being investigated for use in immunotherapy, as it is thought to play a role in monocyte differentiation to PD-L1+ macrophages which may lower interleukin-12, increase interleukin-10, and have a consequent suppressive influence on T-cell activation. 44 Current trials evaluating novel immunotherapies are described in Table 4.
Immunological features of HPV-positive HNSCC suggest that this tumor type has a high potential for responding to immunotherapy, especially with immune checkpoint inhibitors. Given data showing T-cell infiltration in HPV-positive HNSCC, a different approach-one that focuses on checkpoint inhibition-may be beneficial for these patients.
Conclusions
The results of clinical trials demonstrating response to checkpoint inhibitors in HPV-positive head and neck tumors are promising. Late phase clinical trials are exploring whether the use of immunotherapy in HPV-associated tumors will allow de-intensification cytotoxic therapies. Despite encouraging responses and improved cancer survival rates with checkpoint inhibitors, a significant proportion of patients are still being treated with them and will not respond. Hence, additional predictive biomarkers are needed to differentiate the patients who will respond to immunotherapy. In addition, there is a need for newer immunotherapeutic agents that will improve responses in this difficult-to-treat patient population.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590.
2. Andersen AS, Koldjaer Sølling AS, Ovesen T, Rusan M. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int J Cancer. 2014;134(12):2755-2763. doi: 10.1002/ijc.28411.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. doi: 10.3322/caac.21332.
4. Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identities a subset of human papillomavirus-associated oropharyngeal cancers with a favorable prognosis. J Clin Oncol. 2006;24(5):736-747.
5. Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009; 27(12):1992-1998. doi: 10.1200/JCO.2008.20.2853.
6. Pipkorn P, Sinha P, Kallogjeri D, et al. Outcomes of relapsed human papillomavirus-related oropharyngeal squamous cell carcinoma treated with curative intent. Head Neck. 2019;41(5):1312-1319. doi: 10.1002/hed.25557.
7. Dang R, Le V, Miles B, et al. Clinical outcomes in patients with recurrent or metastatic human papilloma virus-positive head and neck cancer. Anticancer Res. 2016;36(4):1703-1709.
8. Baxi S, Fury M, Ganly I, Rao S, Pfister DG. Ten years of progress in head and neck cancers. J Natl Compr Canc Netw. 2012;10(7):806-810. doi: 10.6004/jnccn.2012.0084.
9. Israr M, Rosenthal D, Frejo-Navarro L, DeVoti J, Meyers C, Bonagura VR. Microarray analysis of human keratinocytes from different anatomic sites reveals site-specific immune signaling and responses to human papillomavirus type 16 transfection. Mol Med. 2018;24(1):23. doi: 10.1186/s10020-018-0022-9.
10. Bauman J, Wirth L. Human papillomavirus and oropharyngeal squamous cell carcinoma of the head and neck: a growing epidemic. Adolesc Med State Art Rev. 2014;25(2):489-501.
11. Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167(10):714-724. doi: 10.7326/M17-1363.
12. Bertoli C, Skotheim J, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14(8):518-528. doi: 10.1038/nrm3629.
13. Vokes EE, Agrawal N, Seiwert TY. HPV-associated head and neck cancer. J Natl Cancer Inst. 2015;107(12):djv344. doi: 10.1093/jnci/djv344.
14. D’Souza G, Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med. 2011;53(suppl 1):S5-S11. doi: 10.1016/j.ypmed.2011.08.001.
15. Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467-475. doi: 10.1158/1055-9965.EPI-04-0551.
16. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217.
17. Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576-582. doi: 10.1038/nature14129.
18. Solomon B, Young RJ, Bressel M, et al. Prognostic significance of PD-L1+ and CD8+ immune cells in HPV+ oropharyngeal squamous cell carcinoma. Cancer Immunol Res. 2018;6(3):295-304. doi: 10.1158/2326-6066.CIR-17-0299.
19. Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123-141. doi: 10.1016/j.coms.2014.01.001.
20. Mandal R, ÅenbabaoÄlu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1(17):e89829. doi: 10.1172/jci.insight.89829.
21. Pipkin, ME, Sacks, JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32(1):79-90. doi: 10.1016/j.immuni.2009.11.012.
22. Gildener-Leapman N, Ferris RL, Bauman JE. Promising systemic immunotherapies in head and neck squamous cell carcinoma. Oral Oncol. 2013;49(12):1089-1096. doi: 10.1016/j.oraloncology.2013.09.009.
23. Leibowitz MS, Andrade Filho PA, Ferrone S, Ferris RL. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother. 2011;60(4):525-535. doi: 10.1007/s00262-010-0961-7.
24. Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):744-752. doi: 10.1016/j.tripleo.2009.12.015.
25. Smola S, Trimble C, Stern PL. Human papillomavirus-driven immune deviation: challenge and novel opportunity for immunotherapy. Ther Adv Vaccines. 2017;5(3):69-82. doi: 10.1177/2051013617717914.
26. Jin H-T, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17-37. doi: 10.1007/82_2010_116.
27. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345-1256. doi: 10.1056/NEJMx180040.
28. Reck M, RodrÃguez-Abreu D, Robinson AD, et al; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1 positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774.
29. Motzer RJ, Escudier B, McDermott DF, et al; CheckMate 025 Investigators. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813. doi: 10.1056/NEJMoa1510665.
30. Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731-e741. doi: 10.1016/S1470-2045(17)30607-1.
31. Outh-Gauer S, Alt M, Le Tourneau C, et al. Immunotherapy in head and neck cancers: A new challenge for immunologists, pathologists and clinicians. Cancer Treat Rev. 2018;65:54-64. doi: 10.1016/j.ctrv.2018.02.008.
32. Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 119(2):153-159. doi: 10.1038/s41416-018-0131-9.
33. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45-51. doi: 10.1016/j.oraloncology.2018.04.008.
34. Cohen EEW, Soulières D, Le Tourneau C, et al; KEYNOTE-040 Investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156-167. doi: 10.1016/S0140-6736(18)31999-8.
35. Burtness B, Harrington, KJ, Greil R, et al; KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi: 10.1016/S0140-6736(19)32591-7.
36. Huerter MM, Ganti AK. Predictive biomarkers for immune checkpoint inhibitor therapy: we need to keep searching. J Thorac Dis. 2018;10(suppl 18):S2195-S2197. doi: 10.21037/jtd.2018.06.144.
37. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930-2940. doi: 10.1172/JCI91190.
38. Chow LQM, Haddad R, Gupta S, et al. Antitumor Activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838-3845. doi: 10.1200/JCO.2016.68.1478.
39. Hanna GJ, Liu H, Jones RE, et al. Defining an inflamed tumor immunophenotype in recurrent, metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2017;67:61-69. doi: 10.1016/j.oraloncology.2017.02.005.
40. Haddad RI, Seiwert TY, Chow LQM, et al. Genomic determinants of response to pembrolizumab in head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. 2017;35(15 suppl):6009. doi: 10.1200/JCO.2017.35.15_suppl.6009.
41. Hanna GJ, Lizotte P, Cavanaugh M, et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight. 2018;3(4):e98811. doi: 10.1172/jci.insight.98811.
42. Saleh K, Eid R, Haddad FG, Khalife-Saleh N, Kourie HR. New developments in the management of head and neck cancer- impact of pembrolizumab. Ther Clin Risk Manag. 2018;14:295-303. doi: 10.2147/TCRM.S125059.
43. Andre P, Denis C, Soulas C, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731-1743.e13. doi: 10.1016/j.cell.2018.10.014.
44. Van Esch EM, van Poelgeest MI, Trimbos JB, Fleuren GJ, Jordanova ES, van der Burg SH. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV-induced vulvar neoplasia. Int J Cancer. 2015;136(4):E85-E94. doi:10.1002/ijc.29173.
