Immunogenic Marker May Improve Breast Cancer Detection
ATLANTA-An immunogenic marker, TA-90, can detect breast cancers missed by mammograms and other known markers, Rishab K. Gupta, PhD, said at the Era of Hope: U.S. Department of Defense Breast Cancer Research Program Meeting.
ATLANTAAn immunogenic marker, TA-90, can detect breast cancers missed by mammograms and other known markers, Rishab K. Gupta, PhD, said at the Era of Hope: U.S. Department of Defense Breast Cancer Research Program Meeting.
TA-90, the 90kD glycoprotein tumor-associated antigen, also has shown promising results in tests with melanoma, lung, prostate, ovarian, and colon cancers, Dr. Gupta said in an interview with ONI.
He estimates that 75% to 80% of breast and melanoma tumors would test positive with a TA-90-specific immune complex (IC) detection assay, based on studies conducted at the John Wayne Cancer Institute at the St. Johns Health Center, Santa Monica, California.
Dr. Gupta is director of immunodi-agnosis and vice president for education at the John Wayne Cancer Institute. Donald L. Morton, MD, medical director and surgeon-in-chief, also was one of the authors.
The presence of this antigen in the blood can identify patients who have early-stage cancer or residual cancer after all visible cancer cells are gone, Dr. Gupta commented. If preliminary results are validated in clinical trials, he predicted that a routine blood test for the marker might become a useful adjunct to mammography.
As described by Dr. Gupta, the search for an antigen-based tumor marker began with the hypothesis that the body would release the same amount of antigen whether the tumor cells were together in a nodule or scattered.
In theory, Dr. Gupta said, testing for the marker would detect cancer cells that were not in a mass large enough to be picked up by mammography or other scanning methods.
Monoclonal Antibody Developed
After documenting the presence of TA-90 in blood samples from cancer patients, the researchers developed a murine mo-noclonal antibody to TA-90, and then used the antibody to develop the TA-90-IC assay used in the studies.
In one study, Dr. Guptas group matched serum samples from 106 breast cancer patients with samples from 107 healthy controls. Significantly more of the breast cancer patients63% compared to 2.8% of the control groupscored above a 0.410 ELISA value that the researchers set as a cut-off level for presence of the marker.
A retrospective study focused on 128 patients from a database of breast cancer patients who were positive for TA-90 before surgery. Serum samples were obtained 2 to 12 weeks after surgical resection and tested for TA-90.
Fifty-two patients (41%) remained positive for TA-90 after surgery (two consecutive serum samples above the 0.410 cut-off). Among 43 patients who later had a recurrence, 34 (79%) tested positive for the marker when they were clinically disease free after the initial surgery. Tests were negative for the marker in 67 (79%) of 85 patients who remained disease free.
While TA-90-IC did not catch all cases, Dr. Gupta predicted that the marker would be useful in identifying patients who need further treatment.
If I know the tumor is expressing this marker, and the tumor is taken out by surgery, and the blood is still positive, then I can say the patient still has the cancer in the body somewhere, he explained. Whatever was done by surgery was not enough.
Adjunct to Mammography
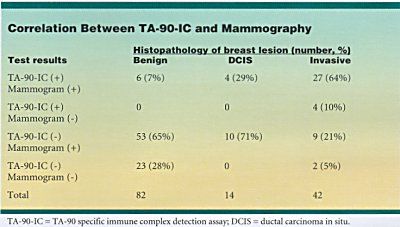
TA-90-IC testing brought the detection rate to 96% of breast cancers (54 of 56) when it was combined with mammography in a prospective study of 138 patients (see table at right). Mammograms were positive for 50 of 56 patients found to have breast cancer at biopsy. TA-90-IC identified four of those who escaped detection by mammography. Only two patients (5%) slipped through both screens.
Dr. Guptas group also compared TA-90 screening with tests for two other tumor markers, CEA and CA15-3, in 68 patients known to have breast cancer. Results showed 81% to be positive for TA-90, 24% for CEA, and 34% for CA15-3. When all three tests were used in concordance, they showed abnormalities in 91% of the patients.
I dont know a single marker good enough to tell everything, but this is certainly a step in the right direction, Dr. Gupta said, adding that he hoped to continue working toward clinical trials. To validate these kinds of findings, we need to have thousands of patients.