Immunotherapy in Castration-Resistant Prostate Cancer: Integrating Sipuleucel-T Into Our Current Treatment Paradigm
Prostate cancer is the second-leading cause of death in men in the United States; more than 217,730 new cases were expected to be diagnosed in 2010.[1] Although the majority of patients with advanced prostate cancer have an initial response to androgen deprivation, essentially all patients eventually progress to a castration-resistant state, manifested by rising levels of prostate-specific antigen (PSA),
ABSTRACT: The availability of several novel antibodies, coupled with viral, DNA, and dendritic-cell vaccines, has renewed interest in immunotherapeutic approaches to the treatment of advanced prostate cancer. Although promising, none of these approaches have led to major clinical activity, and in the case of cell-based immunotherapy with GVAX, new concerns about safety arose when this therapy was used in the castration-resistant setting. A more attractive yet toxic approach has also utilized a check-point blockade with CTLA-4 antibodies. Although initial clinical efficacy has been observed, toxicity appears to be the major limitation of its use in prostate cancer. Sipuleucel-T (Provenge) is an autologous active cellular immunotherapy product that includes autologous dendritic cells pulsed ex vivo with PAP2024, a recombinant fusion protein made of prostatic acid phosphataase (PAP) and granulocyte–macrophage colony-stimulating factor (GM-CSF). Despite the lack of objective anti-tumor activity seen with sipuleucel-T, a recently reported phase III trial demonstrated a significant improvement in the overall survival of men with asymptomatic, minimally symptomatic metastatic castration-resistant prostate cancer (CRPC). This agent is the first FDA-approved novel immunotherapeutic compound for the treatment of a solid malignancy. A better understanding of how clinicians should incorporate this novel agent into the current management of CRPC is needed.
Introduction
Prostate cancer is the second-leading cause of death in men in the United States; more than 217,730 new cases were expected to be diagnosed in 2010.[1] Although the majority of patients with advanced prostate cancer have an initial response to androgen deprivation, essentially all patients eventually progress to a castration-resistant state, manifested by rising levels of prostate-specific antigen (PSA), progressive disease on imaging studies, and/or worsening of symptoms-all in the setting of an anorchid testosterone level.[2] Historically, patients were offered a variety of secondary hormonal manipulations followed by palliative chemotherapy with docetaxel (Taxotere)-based regimens. Following a long period in which there were limited therapeutic developments, the FDA recently approved cabazitaxel (Jevtana), a second generation taxane for patients with disease progression following docetaxel. In addition, recently presented evidence of improved survival for patients receiving the selective CYP-17 inhibitor abiraterone following docetaxel-based chemotherapy will likely result in the approval of another agent in this setting.
A better understanding of the role of inflammation and immune activation in prostate cancer, coupled with the long natural history of the disease,[4] its ability to induce auto-antibodies,[5] and the availability of several tissue-specific proteins that may serve as prostate tumor antigens,[6,7] has facilitated the development of several immune-based therapeutic strategies.[8] In this review, we summarize recent clinical developments in prostate cancer immunotherapy, and we specifically review the clinical development of sipuleucel-T (Provenge), a novel immunotherapeutic agent recently approved by the FDA for the management of asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (CRPC).
Cytokines
Granulocyte–macrophage colony-stimulating factor (GM-CSF) is a small secreted polypeptide that binds to specific cell surface receptors; it is a potent pleiotropic cytokine capable of enhancing hematopoietic differentiation and activation from several lineage precursors, including phagocytic macrophages and dendritic cells.[9] This non-specific activation of antigen-presenting cells (APCs) results in increased co-stimulatory molecule expression on host bone marrow–derived APCs. When administered in vivo, GM-CSF promotes the growth and antigen-presenting capabilities of dendritic cells, leading to T-cell cross-priming.[10] The therapeutic effect of recombinant GM-CSF as an anticancer agent has been studied in several models. Injection of a murine tumorigenic T-leukemia cell line expressing mGM-CSF into pre-established tumors of syngeneic mice led to a significant regression of the tumors.[11] Additionally, mice injected with melanoma cells transfected with a recombinant GM-CSF gene either completely rejected the tumor cells or developed tumors with a mean volume 50 times smaller than in controls.[12] Furthermore, vaccination with GM-CSF-expressing MPC11 cells has been shown to induce a potent antitumor cytotoxic T-lymphocyte response associated with tumor rejection in the majority of the animals tested.[13] Additional studies of autologous or allogeneic prostate tumor cells transfected to produce GM-CSF have demonstrated the generation of potentially relevant immune responses.[11,12]
Exogenous administration of GM-CSF has been studied in men with advanced prostate cancer.[14-16] Although the dose and schedule of administration has varied among clinical trials, it is clear that GM-CSF administration can lead to declines in PSA levels in patients with hormone-nave and castration-resistant disease. However, these declines are often transient, since disease eventually progresses either serologically or radiographically in all patients. The addition of maintenance therapy to GM-CSF also appears to increase the rate of PSA reduction, with no evidence that GM-CSF interferes with PSA measurement or secretion. Although the clinical significance of such PSA changes remains uncertain, these data suggest a potential biologic effect of GM-CSF on PSA. This observation alone is important, since many vaccination strategies have used GM-CSF as an adjuvant. However, the confounding effect that GM-CSF may have on PSA levels makes interpretation of studies that utilize GM-CSF somewhat problematic.
CTLA-4–Based Therapy
In recent years, cytotoxic T-lymphocyte (CTL)-associated antigen-4 (CTLA-4)-targeted therapies have undergone the most extensive evaluation in multiple solid tumors, including prostate cancer. T-cell activation is dependent on the ability of the T-cell receptor to recognize specific antigen-bound major histocompatibility complex (MHC) peptides. Additional antigen-independent costimulatory signals are required for the generation of a T-cell response. These include the normal interaction between CD28 and CTLA-4, both costimulatory receptors located on the surface of T cells, and costimulatory molecules B7-1 (CD80) and B7-2 (CD86), present on APCs. While ligand engagement by CD28 activates T cells, the binding of CTLA-4 and B7 sends an inhibitory signal that down-regulates T-cell activation and serves as a natural brake, altering downstream cytokine production and cell-cycle proliferation, both required for growth.[17-19] In vivo inhibition of CTLA-4 and its ligands through the use of a neutralizing antibody has been shown to induce antitumor activity in several murine tumor models.[20-22] Therefore, blockade of CTLA-4 may represent an important mechanism for potentiating T-cell immunity and anti-tumor response.
Ipilimumab and tremelimumab are two humanized anti-CTLA-4 antibodies that are the lead compounds in the investigation of this immune approach in solid tumors. Small et al evaluated ipilimumab both as monotherapy and in combination with GM-CSF in men with CRPC.[23,24] In the monotherapy trial, 14 patients received ipilimumab as a single dose of 3 mg/kg IV. Treatment was well tolerated, with clinical autoimmunity limited to one patient who developed grade 3 rash/pruritus that required systemic corticosteroids. Although a trend for an increasing percentage of CD4 and CD8 T cells co-expressing MHC class II was observed, flow cytometry analysis of lymphocyte sub-populations failed to demonstrate polyclonal T-cell activation.[23] A subsequent study evaluated the combination of CTLA-4 blockade and GM-CSF in 24 patients with CRPC.[24] While GM-CSF was given as a fixed dose of 250 μg/m²/day for 14 days, ipilimumab was dose-escalated from 0.5 mg/kg to 3 mg/kg, given once every 4 weeks in a sequential phase I design. Observed toxicity was similar to that seen in previous CTLA-4 studies, with 4 patients developing grade 3 adverse events, including a cerebrovascular accident, skin rash, pan-hypopituitarism, temporal arteritis, and diarrhea. Of interest, two of these patients were among those who obtained the greatest clinical benefit, as assessed either radiographically or by PSA level. Immune-based studies have also suggested the enhancing effects of this combination on the activation of circulating CD8+ T cells. Although disappointing clinical results have been observed in other solid tumors in studies using similar antibodies,[25] the recent overall survival (OS) results from a phase III study of ipilimumab with or without a gp100 peptide vaccine in patients with metastatic melanoma[26] clearly suggest that when the appropriate antigen is selected, antibody-mediated therapy has significant potential to become an an important therapeutic strategy in solid malignancies. Although the ideal antigen for prostate cancer immunotherapy has not been well defined, the clinical experience with ipilimumab in melanoma provides impetus for continued immune-based research in prostate cancer.
DNA, Viral, and Cell-Based Vaccines
Several vaccine approaches have undergone clinical evaluation in men with prostate cancer.
One approach involves DNA-based constructs that ultimately activate a specific antitumor immune response. An advantage of this approach is that PAP is a unique antigen in prostate cancer cells, and unlike other vectors (eg, virus), DNA can be rapidly and precisely synthesized. When contrasted with viral vectors, one of the drawbacks of using DNA as a vector is its low level of immunogenicity.[27] Initial studies in rat models using large and repetitive doses of a GMP-grade plasmid DNA vaccine encoding human PAP (pTVG-HP) demonstrated the ability of this vaccine to elicit effective PAP-specific CD4 and CD8 T cells.[28] In a subsequent phase I/II study, 22 prostate cancer patients with biochemical recurrence-only disease were treated in a dose-escalation trial with 100 μg, 500 μg, or 1,500 μg of TVG-HP plasmid DNA, co-administered intradermally with 200 μg of GM-CSF as a vaccine adjuvant, six times at 14-day intervals. Although no major changes in PSA levels were reported, treatment was well tolerated, with minimal if any adverse events, and a significant PAP-specific CD4+/CD8+ T-cell proliferation that included interferon gamma (IFN γ)-secreting CTL activity was observed.[29]
FIGURE 1
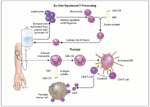
The diagram illustrates the two steps involved in sipuleucel-T therapy.
Using viral or bacterial vectors as a vehicle for vaccine delivery is a cost-effective way to allow the insertion of multiple genes for tumor-associated antigens. Of note, some of these vectors can cause direct local inflammation, which in turn can increase APC recruitment and antigen presentation. Poxviruses have been employed as a vehicle for prostate cancer vaccines in various combinations. Vaccination with recombinant vaccinia virus expressing human PSA (rV-PSA) has been shown to be safe and to induce PSA-specific T-cell responses in animal models and in humans.[30-32]
A phase II trial of the Eastern Cooperative Oncology Group (ECOG) evaluated a heterologous prime/boost vaccination schedule with vaccinia and fowlpox viruses expressing human PSA, in men with BCR-only disease.[33] Patients were randomly assigned to receive four vaccinations with fowlpox-PSA (rF-PSA), three rF-PSA vaccines followed by one rV-PSA vaccine, or one rV-PSA vaccine followed by three rF-PSA vaccines. The primary endpoint was PSA response at 6 months. There were minimal adverse events and no autoimmune events, and almost half of the patients (45.3%) remained free from PSA progression at 19.1 months of clinical follow-up. An increase in PSA-specific T-cell precursors was observed in 46% of patients; however, none of the men in the study had evidence of an anti-PSA antibody response.
PROSTVAC, a construct of fowlpox and vaccinia vectors that contains a triad of costimulatory molecule transgenes (intercellular adhesion molecule 1, B7-1, and leukocyte function associated antigen 3) has also been studied in prostate cancer. Two phase I studies demonstrated the safety and immune effects of this compound in patients with CRPC.[34,35] In a subsequent randomized double-blind phase II study, 125 patients with minimally symptomatical metastatic CRPC were randomly assigned in a 2:1 fashion to receive either PROSTVAC-VF plus GM-CSF or empty vectors plus saline injections. Although no difference in the primary endpoint of progression-free survival (PFS) was found (3.8 vs 3.7 months; hazard rate [HR], 0.88; P = .60), patients treated with PROSTVAC-VF had a superior OS compared with the OS of those in the control arm (25.1 vs 16.6 months; HR, 0.56; P = .0061).[36] The toxicity was similar to that seen with other viral vectors tested in prostate cancer. Grade 1 and 2 injection site reactions, fatigue, fever, and nausea were the most common adverse events reported. No detectable antibody response to PSA was observed, and all patients had an augmented antibody response to the vaccinia and fowlpox vectors. There was no association between the antivector antibody production and OS. A pivotal phase III trial of PROSTVAC-VF is currently planned.
The use of allogeneic prostate cancer cells as immunotherapy vectors has been another attractive immunotherapeutic approach in advanced prostate cancer. GVAX is a combination of two prostate carcinoma cell lines (LNCap and PC-3) modified with the GM-CSF gene and thereby engineered to increase the antigen-presenting capabilities of dendritic cells to T cells.[37-40] Two subsequent phase II studies evaluating the clinical activity and safety of GVAX in men with CRPC[41,42] demonstrated an improvement in the median OS compared with historical data from the Halabi nomogram, a validated pre-treatment prognostic model for CRPC.[43] In the first trial, Small et al[41] reported that the median OS for GVAX-treated patients was 26.2 months, compared with the 19.5 months (P = .01) predicted by the nomogram. The trial by Higano et al,[42] which employed a vaccine construct that was re-engineered to secrete a higher dose of GM-CSF, also demonstrated a superior OS benefit (35 months) compared with the same historical control. When the immune effects of treatment were evaluated, immunoreactivity was found not to be a significant predictor of survival or clinical response. The most common adverse events reported in both studies were injection site reactions and flu-like symptoms.
On the basis of these results, two large randomized phase III trials were launched. The first (VITAL-1) was a head-to-head comparison of GVAX vs docetaxel/prednisone, and the second (VITAL-2) was a combination of docetaxel and GVAX vs docetaxel/prednisone. Although VITAL-1 completed its target accrual of 600 patients with CRPC, VITAL-2 was halted after an early interim analysis demonstrated an imbalance in the number of deaths in the immunotherapy arm (67 vs 47 in the docetaxel/prednisone arm). On the basis of this finding, an interim analysis for VITAL-1 was conducted. There was no OS difference between GVAX and docetaxel/prednisone (20.7 and 21.7 months, respectively; HR, 1.03; P = 0.78).[44] To date, the relationship between GVAX and docetaxel treatment and deaths remains unknown. Based on these results, further clinical development of GVAX for prostate cancer has been terminated.
Dendritic Cell–Based Approaches
Sipuleucel-T is an autologous active cellular immunotherapy product that consists of autologous peripheral blood mononuclear cells (PBMCs) pulsed ex vivo and activated in vitro with a recombinant fusion protein (PA2024). The recombinant fusion protein, PA2024, is composed of a full-length PAP linked via its COOH terminus to the NH2 terminus of full-length GM-CSF.[45, 46] When administered in vivo, GM-CSF promotes the growth and antigen-presenting capabilities of dendritic cells, leading to T-cell cross-priming.[10] The preparation of sipuleucel-T involves a standard leukapheresis of approximately 1.5 to 2.0 total blood volumes with isolation of PBMCs through density-gradient centrifugation to allow for the removal of platelets and monocytes. The product is then incubated with the recombinant fusion protein PAP2024. After the desired clinical dose has been formulated, the final product is then transported to the respective facility and infused into the patient within 8 hours of formulation. Multiple phase I/II sequential trials evaluated the clinical and immune effects of sipuleucel-T in patients with CRPC. In a trial that included 31 patients with metastatic or non-metastatic CRPC, treatment with sipuleucel-T was administered in weeks 0, 4, and 8, with a fourth infusion in week 24 only to those patients whose disease was stable or better after the initial three infusions. In all patients receiving sipuleucel-T, a T-cell response developed that was defined by the immune response to the recall antigen influenza and to the naive antigen KLH (keyhold limpet hemocyanin). Antibodies to PAP and GM-CSF were also evaluated by specific enzyme-linked immunosorbent assay (ELISA) on serum samples obtained at baseline and then every 4 weeks. None of the patients had pre-existing antibodies to PAP, whereas after treatment, 16 of 31 patients (52%) had antibodies.[47]
Three small prospective phase II studies that included patients with castrate-resistant and androgen-dependent, biochemically relapsed disease initially demonstrated the safety and modest clinical activity of sipuleucel-T in prostate cancer. The first trial, which included 19 men with CRPC, demonstrated a modest PSA response in 2 of the 19 patients (PSA decline > 50% compared with baseline) and no objective disease response in those with Response Evaluation Criteria in Solid Tumors (RECIST)-defined measurable disease. In terms of immune parameters, T-cell responses to PAP2024 were elicited in all evaluated patients; the responses lasted for the entire duration of the clinical trial.[48] Similarly, two small single-institution studies demonstrated that treatment with sipuleucel-T alone[49] or in combination with bevacizumab (Avastin), a monoclonal humanized antibody against all active isoforms of vascular endothelial growth factor (VEGF),[50] leads to an increase in PSA doubling time in patients with androgen-dependent, biochemically relapsed prostate cancer. While the study of sipuleucel-T monotherapy demonstrated a median increase in PSA doubling time of 62% (4.9 months before treatment vs 7.9 months after treatment; P = .09; signed-rank test), the median increase in PSA doubling time when bevacizumab was added to sipuleucel- T appeared to be greater than 150% (6.9 months vs 12.7 months; P = .01). Although no immune parameters were reported in the monotherapy trial, all patients in the combination study with bevacizumab demonstrated induction of an immune response against sipuleucel-T.
The phase III development program for sipuleucel-T included 2 parallel randomized, double-blind, placebo-controlled studies, D9901 and D9902A.[51,52] D9901 was a multi-institution double-blind, placebo-controlled, randomized phase III trial designed to test the effect of sipuleucel-T on time to progression (TTP) and OS in 127 men with asymptomatic metastatic CRPC. Patients in this trial were randomized in a 2:1 ratio to receive 3 infusions of sipuleucel-T (n=82) or placebo (n=45) every 2 weeks. On disease progression, placebo patients received a similar product made with frozen leukapheresis cells. D9902A was an identical study to D9901 but was prematurely stopped after the initial TTP results from D9901 were reported. Thus, the study was underpowered; it ultimately enrolled only 98 patients.
The primary endpoint of both studies was TTP. Imaging studies were completed every 8 weeks until week 32, then every 12 weeks thereafter, and progression was confirmed by independent review. PSA was not used to determine disease progression or to trigger radiographic evaluations. The median TTP for patients receiving sipuleucel-T in study D9901 was 11.7 weeks, compared with 10.0 weeks for placebo (P = .052; HR, 1.45). Similarly, the TTPs in study D9902A were 10.9 and 9.9 weeks, respectively (P =.719; HR, 1.09). Of the 147 patients from both trials who received sipuleucel-T, only 5 had a PSA reduction of > 50%; the overall PSA response rate was 4.8%. No objective responses were observed in those patients with measurable disease who received sipuleucel-T.
While neither study was powered to determine the impact of sipuleucel-T on OS, a pre-planned 3-year OS assessment was carried out. The median OS in the D9901 study was 25.9 months for sipuleucel-T–treated patients and 21.4 months for those who received placebo (P = .01; HR, 1.70). Although no survival benefit was observed in the D9902A trial (19 months vs 15.7 months; P = .331),[52] when both trials were integrated together, the median OS was 23.2 months for sipuleucel-T and 18.9 months for placebo (P = .011). Similarly, the percentage of patients alive at 36 months was 33% for those who received sipuleucel-T and 15% for those who received placebo. The OS benefit found in these studies was maintained after adjusting for multiple predefined CRPC prognostic factors,[53,54] including the subsequent use of docetaxel-based chemotherapy (P = .022).[52] Also provocative was the strong correlation between CD54 up-regulation and survival (P = .009), observed in both studies. CD54, also known as intracellular adhesion molecule 1, is expressed on dendritic cells and plays an important role in the synapse between dendritic cells and T cells.
To confirm the survival impact of sipuleucel-T, 512 men with metastatic CRPC were randomized (in a 2:1 ratio) in a phase III, double-blind, placebo-controlled, multicenter trial called the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) study.[53] Eligibility for this trial was similar to that in previous CRPC studies.[51,52] All patients were stratified according to Gleason grade, number of bone metastases, and bisphosphonate use. Progressive disease (PD) was independently monitored using PSA and imaging studies at weeks 6, 14, 26, and 34, and every 12 weeks thereafter. During the trial, placebo patients in whom PD developed could enroll in an open-label salvage protocol and receive APC8015F, a product manufactured according to the same specifications as sipuleucel-T from cells cryopreserved at the time the placebo was prepared.
Survival was the primary endpoint of the study, and it was analyzed on the basis of a stratified Cox regression model, with adjustment for the natural logarithm of the baseline levels of PSA and lactate dehydrogenase and stratified according to randomization factors.[54,55] The median OS was 25.8 months for sipuleucel-T–treated patients and 21.7 months for patients who received placebo, with an adjusted HR for death of 0.78 (95% confidence interval [CI], 0.61 to 0.98), which represents a relative reduction in the risk of death of 22% (P = .03). Similar results were obtained with the use of the unadjusted, stratified model and the log-rank test (HR, 0.77; 95% CI, 0.61 to 0.97; P = .02). The reduction in the risk of death from prostate cancer in the sipuleucel-T group (HR, 0.77; 95% CI, 0.61 to 0.98; P = .04) was similar to the reduction in the risk of death from any cause. There was no difference in the median time to objective PD (3.7 months in the sipuleucel-T group vs 3.6 months in the placebo group; HR, 0.95; 95% CI, 0.77 to 1.17; P = .63). Similar results were observed for the time to clinical PD (HR, 0.92; 95% CI, 0.75 to 1.12; P = .40). Only one patient who received sipuleucel-T achieved a partial remission; 2.6% had a PSA decline of < 50%. The estimated effect of sipuleucel-T treatment in those patients who received subsequent docetaxel-based chemotherapy was consistent with the results of the primary efficacy analysis (HR for death, 0.78; 95% CI, 0.62 to 0.98; P = .03).
With regard to immune changes during the study, titers of antibodies against the immunizing antigen PA2024 at any time after baseline were observed in 100 of 151 patients (66.2%) in the sipuleucel-T group and in 2 of 70 patients (2.9%) in the placebo group. Similarly, titers of antibodies against PAP were observed in 43 of 151 patients (28.5%) in the sipuleucel-T group and in 1 of 70 patients (1.4%) in the placebo group. At week 6 of treatment, T-cell proliferation responses (stimulation index, >5) to PA2024 were observed in 73% of patients in the sipuleucel-T group compared with 12% in the placebo group. No changes in the immune correlative studies conducted in this trial correlated with outcome.
Overall, therapy with sipuleucel-T is well tolerated. Almost all patients in the IMPACT trial received all three planned infusions, and no patient withdrew from the study secondary to toxicity. The most common toxicities were grade 1 and grade 2 and included chills, fever (pyrexia), headache, influenza-like illness, myalgia, hypertension, hyperhidrosis, and groin pain. Most of these adverse events occurred within 1 day after infusion and resolved within 1 to 2 days. Among patients in the sipuleucel-T group, grade 3 events that were reported for at least 1 patient within 1 day after infusion were chills (in 4 patients), fatigue (in 3 patients), and back pain, hypertension, hypokalemia, and muscular weakness (in 2 patients each); one grade 4 event was reported (intravenous catheter–associated bacteremia).
Discussion
The management of CRPC has evolved slowly over the past decade. The evolution of docetaxel-based chemotherapy as a standard of care has been notable but until recently was an isolated therapeutic development. The recent FDA approval of sipuleucel-T and cabazitaxel,[56] and the anticipated approval of abiraterone,[57] will over a very short period of time add three new agents to the treatment armamentarium, all of which independently have improved OS in prostate cancer.
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Abiraterone
Bevacizumab (Avastin)
Cabazitaxel (Jevtana)
Docetaxel (Taxotere
Gefitinib (Iressa)
Gp100 peptide vaccine
GVAX
Ipilimumab
Prednisone
PROSTVAC-VF
Sipuleucel-T (Provenge)
Tremelimumab
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Unlike cabazitaxel and abiraterone, which in addition to a survival advantage have clear evidence of objective anti-tumor activity (as documented by both response rates and impact on PFS), sipuleucel-T’s demonstrated ability to improve OS occurs without evidence of a measurable anti-tumor effect (TTP, objective response rate, and serologic response [PSA reduction]). Some clinicians find it somewhat perplexing that despite the lack of tumor burden reduction when measurable disease was present, treatment with sipuleucel-T was nonetheless able to impact the natural history of this disease in a positive manner. Although sensitivity analyses in the IMPACT trial demonstrated that subsequent treatment (mostly docetaxel-based chemotherapy) did not account for the survival difference observed, the study design was not powered to detect such a difference.
An especially intriguing-but still unanswered-question is what the true mechanism of action of sipuleucel-T is. Therapy with sipuleucel-T has been demonstrated to produce antigen-specific T-cell responses, and patients with elevated antibody titers after treatment appear to have improved survival compared with those without titer elevations. However, these findings require further validation. A better understanding of the immune effects of sipuleucel-T is needed to help improve the selection of patients who are likely to derive the greatest benefit from treatment with this agent.
With the rapid introduction of a number of new agents and the clinical limitations of sipuleucel-T, the question of how to optimally integrate sipuleucel-T into the management paradigm will require additional study and clinical experience. Some early observations can be made in an effort to help guide clinicians in making management choices. Given the absence of direct anti-tumor activity, sipuleucel-T should not be presented to patients as-or utilized as-a “bridge” therapy between treatments (ie, administration of sipuleucel-T will not delay the need for chemotherapy in a patient with a clinical picture of rapidly progressive radiographic disease or with early disease-related symptoms (progressive pain, fatigue, weight loss, etc) With the anticipated approval of abiraterone, the use of corticosteroids in combination with this lyase inhibitor will no doubt raise issues regarding the potential impact of long-term corticosteroid use on the “effectiveness” of sipuleucel-T; there currently is no evidence to guide decision making regarding this vaccine and subsequent use of corticosteroids.
Notwithstanding the challenges involved in the development of immunotherapy for prostate cancer, the regulatory approval of sipuleucel-T has opened the door to multiple opportunities for immune drug development. Future studies will require thoughtful clinical trial designs, including appropriate patient selection and translational and clinical endpoints.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300
2. Oh WK, Kantoff PW. Management of hormone refractory prostate cancer: Current standards and future prospects. J Urol. 1998;1160:1220-9.
3. Chi KN, Bjartell A, Dearnaley D, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol. 2009;56:594-605.
4. Coffey DS, Isaacs JT. Control of prostate growth. Urology. 1981;17:17-24.
5. Wang X, Yu J, Sreekumar A, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005; 353:1224-35.
6. Taylor BS, Varambally S, Chinnaiyan AM. Differential proteomic alterations between localized and metastatic prostate cancer. Br J Cancer. 2006;95:425-30.
7. Rhodes DR, Barrette TR, Rubin MA, et al. Meta-analysis of microarrays: interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res. 2002;62:4427-33.
8. Antonarakis ES, Drake CG. Current status of immunological therapies for prostate cancer. Curr Opin Urol. 2010;20:241-6.
9. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271-96.
10. Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. N Engl J Med. 1992;372:28-35.
11. Nelson WG, Simons JW, Mikhak B, et al. Cancer cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer as vaccines for the treatment of genitourinary malignancies. Cancer Chemother Pharmaco. 2000;46 Suppl:
S567-72.
12. Simons JW, Mikhak B, Chang JF, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160-8.
13. Simons JW, Small ES, Nelson W, et al. Phase II trials of a GM-CSF gene-transduced prostate cancer cell line vaccine (GVAX) demonstrate anti-tumor activity. Program/Proceedings of the American Society of Clinical Oncology. 2001;20:175b (abstract 1073).
14. Small EJ, Reese D, Um B, et al. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738-44.
15. Rini BI, Weinberg V, Bok R, et al. Prostate-specific antigen kinetics as a measure of the biologic effect of granulocyte macrophage colony-stimulating factor in patients with serologic progression of prostate cancer. J Clin Oncol. 2003;21:99-105.
16. Dreicer R, See WA, Klein EA, Phase II trial of GM-CSF in advanced prostate cancer. Invest New Drugs. 2001;19:261-65.
17. Krummel MF, Allison JP. CTL A-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533-40.
18. Greenwald RJ, Boussiotis VA, Lorsbach RB, et al. CT LA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145-55.
19. Egen JG, Kuhns MS, Allison JP. CTL A-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611-8.
20. Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1994;7:445-50.
21. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734-6.
22. Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355-66.
23. Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810-5.
24. Fong L, Kwek SS, O’Brien S, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA-4 blockade and GM-CSF. Cancer Res. 2009;2:609-615.
25. Ribas A, Hauschild A, Kefford R, et al. Phase III, open label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. J Clin Oncol. 2008;26:9011.
26. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
27. Johnson LE, Frye TP, Chinnasamy N, et al. Plasmid DNA vaccine encoding prostatic acid phosphatase (PAP) is effective in eliciting autologous antigen-specific CD8+ T cells. Canc Immunol Immunoth. 2007;56:885-95.
28. Johnson LE, Frye TP, Arnot AR, et al. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP). Vaccine. 2006;3:293-303.
29. McNeel DG, Dunphy EJ, Davies JG, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;25:4047-54.
30. Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632-8.
31. Hodge JW, Schlom J, Donohue SJ, et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer. 1995;63:231-7.
32. Gulley J, Chen AP, Dahut W, et al. Phase 1 study of vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109-17.
33. Kaufman HL, Wang W, Manola J, et al: Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122-32.
34. DiPaola RS, Plante M, Kaufman H, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1-5.
35. Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol 2007;178:1515-1520.
36. Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099-1105.
37. Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granuloctye-macrophage colony-stimulating factor stimulates potent, specific, and long lasting anti-tumor immunity. Proc Natl Acad Sci. 1993;90:3539-43.
38. Jaffee EM, Lazenby A, MeuerJ, et al. Use of murine models of cytokine-secreting tumor vaccines to study feasibility and toxicity issues critical to designing clinical trials. J Immunother Emphasis Tumor Immunol. 1995;18:1-9.
39. Simons JW, Mikhak B, Chang JF, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160-8.
40. Simons JW, Carducci MA, Mikhak B, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer. Clin Cancer Res. 2006;12:3394-401.
41. Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883-91.
42. Higano CS, Corman JM, Smith DC, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;5:975-84.
43. Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with HRPC. J Clin Oncol. 2003;21:1232-7.
44. Higano C, Saad F, Somer B, et al. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC). 2009 ASCO GU Meeting. Abstract LBA150.
45. Haines AM, Larkin SE, Richardson AP, et al. A novel hybridoma antibody (PASE/4LJ) to human prostatic acid phosphatase suitable for immunohistochemistry. Br J Cancer. 1989;60:887-92.
46. Goldstein NS. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am J Clin Pathol. 2002;117:471-7.
47. Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. Clin Oncol. 2000;23:3894-903.
48. Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a phase 2 trial. Prostate. 2004;3:197-204.
49. Beinart G, Rini BI, Weinberg V, Small EJ. Antigen-presenting cells 8015 (Provenge) in patients with androgen-dependent, biochemically relapsed prostate cancer. Clin Prostate Cancer. 2005;1:55-60.
50. Rini BI, Weinberg V, Fong L, et al. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (Provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006;1:67-74.
51. Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;19:3089-94.
52. Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;16:3670-9.
53. Kantoff PW, Higano CS, Shore ND, et al, for the IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22
54. Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;19:3972-82.
55. Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;7:1232-7.
56. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;9747:1147-54.
57. De Bono JS, Logothetis C, Fizazi K, et al. Abiraterone acetate plus low dose prednisone improves overall survival in patients with metastatic castration-resistant prostate cancer (CRPC) who have progressed after docetaxel-based chemotherapy: results of COU-AA-301, a randomized double-blind placebo-controlled phase 3 study. Abstract LBA5; ESMO 2010.