Inflammatory Breast Cancer: What Progress Have We Made?
Over the past decade, inflammatory breast cancer (IBC), a rare and aggressive subtype of locally advanced breast cancer (LABC), has received much attention at the level of public awareness as well as at the level of research.
Inflammatory breast cancer (IBC) is a rare and aggressive subtype of locally advanced breast cancer (LABC). Its diagnosis is primarily clinical; however, a pathological confirmation of invasive cancer is required. Historically, IBC was a uniformly fatal disease. A major advance in the last three decades has been the introduction of a multidisciplinary approach to the management of this aggressive disease, incorporating pre-operative chemotherapy, surgery, and radiation therapy; this approach has significantly improved survival. Our review focuses on the progress made in the field of IBC research over the last decade, with particular attention to advances in the areas of epidemiology, molecular biology, and clinical management.
Over the past decade, inflammatory breast cancer (IBC), a rare and aggressive subtype of locally advanced breast cancer (LABC), has received much attention at the level of public awareness as well as at the level of research. The diagnosis of IBC is primarily clinical, although a pathological confirmation of invasive cancer is required. The most widely used definition of IBC is that which has been put forward by the American Joint Committee on Cancer; this definition states in part that IBC is “a clinicopathologic entity characterized by diffuse erythema and edema of the breast, often without an underlying palpable mass.”[1] The incidence of IBC varies across different geographic locations but typically accounts for approximately 1% to 5% of all newly diagnosed breast cancers in the United States.[2] The rarity of the disease makes large prospective clinical trials difficult, with the result that much of the information we have on IBC is based on retrospective studies. Furthermore, the subjective nature of the clinical diagnostic criteria has led to wide variability in clinical reports at both the epidemiological and molecular levels.
Historically, IBC was a uniformly fatal disease, with a 5-year actuarial overall survival of less than 5%, a median survival of only 15 months,[3] and local recurrence rates as high as 50% when treated with surgery alone or in combination with radiation therapy.[4,5] A major advance of the last three decades has been the introduction of a multidisciplinary approach to the management of this aggressive disease that incorporates pre-operative chemotherapy, surgery, and radiation therapy. As a result, survival outcomes have improved significantly, with 15-year survival rates of 20% to 30% reported.[6,7] However, despite this progress, most women in whom IBC is diagnosed eventually experience a recurrence and die from the disease-highlighting the need for further research.
Specifically, current research is focused on the following objectives:
• Refining the clinical diagnostic parameters to decrease subjectivity.
• Delineating diagnostic markers to enhance diagnostic accuracy.
• Developing predictive and prognostic markers to aid in treatment planning.
• Characterizing IBC at the molecular level.
• Developing targeted therapies based on an enhanced understanding of the biology of IBC, with the hope that such an approach will positively impact prognostic outcomes.
High-throughput molecular analysis has provided great insight into the understanding and characterization of non-IBC breast tumors.[8] This has perhaps been one of the biggest advances in the field of breast cancer. Such technology has rarely been used in the realm of IBC, in part because of IBC's comparative infrequency and thus the small number of diagnostic samples. However, the last decade has seen an upsurge in the understanding of IBC at both a clinical and a molecular level.
Epidemiology and Risk Factors
Epidemiological research on IBC has not enjoyed the kind of progress seen with other subtypes of breast cancer. This lag in progress is primarily due to the rarity of the disease, which has resulted in small numbers of patients with IBC being available to any single institution; it is also due to the lack of an agreed upon case definition for IBC, which has made it difficult for institutions to collaborate. Nonetheless, data have emerged from large population-based studies. One important observation that has been made is that, unlike with non-IBC breast tumors and despite the overall low incidence of IBC (it accounts for only 1% to 5% of all newly diagnosed breast cancer cases), the incidence of IBC in the United States appears to be increasing.[1] This may in part be the result of increased awareness among both patients and oncologists.
It is interesting to note that there is striking geographic variation in the incidence of IBC, with the lowest incidence reported in North America[1] and a higher incidence reported in North African countries-especially Morocco, Algeria, Tunisia, and Egypt, where the incidence of IBC has been reported to be between 10% and 15%.[9] However, it is uncertain in these countries whether case registration is complete and whether strict definitions are used for IBC detection.
Variations in incidence have also been reported among different ethnic groups in the United States. In a large population-based study of 3,626 women with IBC diagnosed between 1994 and 1998, Wingo et al[10] reported an IBC rate of 1.3 per 100,000 for all races combined, with African Amer-ican women having the highest risk (1.6 per 100,000) and Asian and Pacific Islander women having the lowest risk (0.7 per 100,000). Other key observations concerning women in whom IBC has been diagnosed include their younger age at presentation compared with women with non-IBC, their lower median survival compared with patients with LABC (2.9 years vs 6.4 years),[1] and the significantly in-creased odds of IBC developing in women with a high body mass index.[11] Currently, the mammary tumor virus is being investigated as a potential risk factor for IBC based on the observation that in North America a higher incidence of the viral sequence (71%) has been observed in women with IBC than in women with sporadic breast cancers; a similar incidence has also been observed in IBC tumor samples from Tunisia.[12]
Advances in the Molecular Characterization of IBC
Molecular characteristics
As stated earlier, IBC is diagnosed primarily on the basis of a set of clinical characteristics, with pathological confirmation of invasive carcinoma also required. Characteristic pathological findings that can aid in the diagnosis of IBC include the presence of dermal lymphatic invasion by invasive tumor emboli, leading to obstruction of the lymphatic drainage and resulting in a clinical appearance of erythema and edema mimicking an inflammatory process.[13,14] However, the absence of a dermal lymphatic invasion does not preclude a diagnosis of IBC. Molecular alterations that have been reported with high incidence in IBC include negative hormone receptor status,[15] overexpression and/or gene amplification of human epidermal growth factcor receptor 2 (HER2),[16] overexpression of epidermal growth factor receptor (EGFR), high S-phase fraction, high-grade p53 mutation,[16] overexpression of E-cadherin,[17] dysfunction of mucin 1, overexpression of RhoC GTPase (a gene involved in cytoskeletal reorganization),[18] loss of the LIBC (lost in inflammatory breast cancer) gene,[18] and a high level of angiogenesis.[19] Studies have also demonstrated high levels of expression of multiple chemokine receptors in IBC. In a recent study, Cabliogu et al looked at the prognostic implications of the chemokine receptors CXCR4 and CCR7, as well as HER2 and EGFR, in IBC.[20] Working with a sample of 44 cases of IBC, the authors reported high levels of expression of CXCR4, EFGR, and HER2 amplification that were associated with a higher risk of recurrence and death. However, the molecular alterations described here are not specific to IBC. Several studies looking at these molecular markers have shown that in multivariate analysis, IBC status itself still remains an independent adverse prognostic feature; this indicates that other molecular differences are probably present that distinguish IBC from non-IBC.
Gene expression profiling
To better define the differences between IBC and non-IBC that are present at the molecular level, high-throughput molecular technologies have been utilized that allow for the analysis of several thousand genes in a tumor sample simultaneously. Over the past decade, several studies have specifically looked at expression profiling. To the best of our knowledge, six groups have profiled clinical samples of IBC and reported their results.[21-30] One of the earliest publications, from Bertucci et al in 2004,[21] used cDNA microarrays containing approximately 8000 genes to profile 37 IBC and 44 non-IBC prechemotherapy tumor samples. The authors made a number of important observations, including their finding of the presence of extensive transcriptional heterogeneity in IBC, and their demonstration that IBC exhibited overexpression of basal, immune, and vascular gene clusters and exhibited underexpression of luminal gene clusters compared with non-IBC tumors. In addition, they identified a 109-gene signature that discriminated between the two sample types. Using the same data set, Bertucci et al further demonstrated[22] that similar to non-IBC, five molecular subtypes (defined according to the Stanford intrinsic gene set)-including luminal A, luminal B, basal, HER2-positive, and normal-like-could be demonstrated in IBC. Higher frequencies of basal and HER2-positive IBC samples were observed compared with the frequencies of these two subtypes in non-IBC samples.
Van Laere et al in 2005[23] used cDNA microarrays containing approximately 6000 genes to profile 16 IBC and 18 non-IBC prechemotherapy samples. Using supervised analysis, the authors identified 953 genes that exhibited a 1.5-fold difference in expression between the two sample types. Of these genes, 756 (retained after the exclusion of genes related to the expression of estrogen receptor 1 and HER2) resulted in a perfect segregation of IBC and non-IBC samples. Using the same data set, the investigators further identified five different cell-of-origin subtypes in the two sample sets, with the basal (37%) and HER2-positive (13%) subtypes representing 50% of the IBC samples.[24] Using Affymetrix technology on a set of 19 IBC and 40 non-IBC samples, the investigators confirmed their initial findings, identifying 26% of the IBC samples as basal and 42% as HER2-positive.[25]
Biche et al[26] used real-time polymerase chain reaction (RT-PCR) to quantify the mRNA expression level of 538 candidate genes in 8 IBC and 8 non-IBC samples; they demonstrated a more than two-fold change in a set of 48 genes between the two sample types. The investigators further identified a three-gene signature composed of epiregulin (EREG), v-myc myelocytomatosis viral-related oncogene (MYCN), and sonic hedgehog (SHH). This gene signature was shown to distinguish between three subgroups of women with IBC with different recurrence rates.
Nguyen et al,[28] using Affymetrix U133A microarrays, defined the expression profiles of prechemotherapy samples from 13 patients with IBC and 12 patients with stage III non-IBC. Because of considerable heterogeneity across both sample types, a robust signature could not be identified; however, the investigators were able to demonstrate a higher expression of genes associated with increases in metabolic rate, lipid signaling, and cell turnover in the IBC samples than in the non-IBC samples. They also confirmed the presence of three molecular subtypes of IBC, including luminal, HER2-positive, and basal subtypes.
The most recent study comes from the group at the National Cancer Institute in Bethesda;[29] these investigators used Affymetrix U133A microarrays to profile 15 IBC samples and 35 non-IBC samples. Eighty seven percent of the IBC samples and 23% of the non-IBC samples were obtained postchemotherapy, and each sample was obtained via laser capture microdissection. The investigators were unable to identify a significant signature in the epithelia that could distinguish between the two sample types, but they were able to identify a stromal signature that could distinguish IBC from non-IBC. They further demonstrated that the 109-gene signature originally identified by Bertucci et al[21] performed better in classifying IBC when the tumor stroma was used instead of the tumor epithelial; this finding lends evidence to the hypothesis that gene expression in the stroma contributes to the IBC phenotype.
There are several drawbacks to these studies, including the facts that they involved very small numbers of IBC and non-IBC samples, that the definition of IBC varied across studies, that some studies used prechemotherapy samples while others used postchemotherapy samples, and that the methods used to collect samples also varied. Despite these drawbacks, we can derive valuable information from the data described. First, the transcriptional heterogeneity of IBC appears to be as extensive as that for non-IBC. Second, at the clinical level, the existence of molecular subtypes within IBC may in the future prove to have a prognostic significance similar to that seen in non-IBC subtypes. Third, the genetic signatures identified may also be used to divide patients with IBC into several prognostic groups. Bertucci et al[21] identified an 85-gene set that divided patients with IBC into two groups with different pathological complete response rates (pCR), while the three-gene signature identified by Biche et al[26] categorized patients with IBC into good, intermediate, and poor outcome groups. Fourth, a signature identified for IBC may reflect the aggressiveness of breast cancer generally and thus may play a prognostic role if also identified within non-IBC samples. Van Laere et al[30] applied a gene signature predictive of IBC to a set of 1157 non-IBC samples and showed that non-IBC samples with an IBC-like phenotype had a significantly shorter relapse-free survival than samples that did not have an IBC-like phenotype.
A Multidisciplinary Approach to IBC: A Major Advance
Perhaps the most important progress made in the field of IBC has been the adoption of a multidisciplinary approach to its diagnosis and management. The use of improved diagnostic and staging modalities followed by a sequential treatment management approach in the form of pre-operative chemotherapy followed by surgery and radiation therapy has significantly improved the prognostic outcomes associated with this aggressive disease.
Advances in imaging
The past 2 decades have seen a significant improvement in the imaging techniques used in IBC. These improved techniques have played key roles in tumor characterization, thereby facilitating image-guided biopsy, defining the extent of loco-regional disease, diagnosing distant metastases, and playing an important role in the evaluation of response to pre-operative chemotherapy.[31]
The introduction of digital mammography with enhanced contrast resolution has allowed for improved detection of associated IBC-related abnormalities, including skin thickening, trabecular and stromal thickening, and diffuse increased breast density-which are all changes that reflect dermal lymphatic invasion. High-resolution ultrasonagraphy allows for the identification of any focal mass lesion, thereby facilitating image-guided biopsy for assessment of the involvement of loco-regional lymph nodes, which ultimately helps in the planning of locoregional treatment.[32] MRI, when used in the diagnosis of IBC, has been found to have a high sensitivity for detection of the primary breast parenchymal lesion, as well as for global skin abnormalities; MRI can thus help guide high-yield skin punch biopsies. PET/CT has also been investigated as a tool that can potentially help in the diagnosis and staging of IBC. In a large retrospective study of 41 women with IBC that looked at the role of PET/CT as an initial staging tool, Carkaci et al[33] reported hypermetabolic uptake in 98% of the patients. The investigators also noted that PET/CT detected the presence of ipsilateral axillary lymph nodes in 90% of women. Interestingly, 20 patients in this study were found to have distant metastatic disease, 7 of whom were not known to have metastases before they underwent PET/CT. Wang et al[32] looked at the roles of all three imaging modalities in women with IBC. They concluded that breast MRI was the most accurate for detecting primary breast parenchymal lesions, that ultrasonography was useful for detecting the extent of loco-regional lymph node involvement, and that PET/CT was useful for providing additional information on the presence or absence of distant metastatic disease.
Pre-operative chemotherapy
The optimal pre-operative regimen for the management of IBC has yet to be fully delineated due to the fact that information from large prospective clinical trials is not available. Much of the information that is available has been extrapolated from data on patients with non-IBC and from retrospective data on IBC patients. The Early Breast Cancer Trialists’ Collaborative Group’s 15-year update revealed that 6 months of an anthracycline-based polychemotherapy regimen reduced the annual breast cancer death rate by 38% among women younger than 50 years of age and by 20% among women aged 50 to 69 years.[34] One of the first and largest studies to look at the question of anthracyline use in women with IBC comes from the University of Texas M.D. Anderson Cancer Center. The investigators pooled information on 178 women with IBC who were enrolled prospectively in 4 clinical trials; all women received a pre-operative anthracyline-based regimen followed by definitive local treatment.[35] The investigators reported a median overall survival of 40 months, with an impressive 28% of patients being alive and disease-free at 15 years. In a more recent study by Baldini et al,[36] the investigators looked at 68 women with IBC who were enrolled in 2 prospective trials. All women received an anthracycline-based pre-operative regimen followed by definitive local treatment and additional adjuvant chemotherapy. The investigators on this study reported 5- and 10-year overall survival rates of 44% and 32%, respectively.
Taxanes form an integral part of treatment of women with node-positive non-IBC. The role of taxanes has been investigated in women with IBC as well. Cristofanilli et al[37] retrospectively looked at 240 women with IBC enrolled in 6 prospective clinical trials; they specifically looked at the question of whether adding taxanes to an anthracycline-based pre-operative regimen provided benefit. The authors reported that the addition of taxanes resulted in improved median, overall, and progression-free survival; the improvements reached statistical significance, specifically among women with estrogen receptor–negative disease (median overall survival, 54 months vs 32 months in patients who did not receive taxanes; median progression-free survival, 27 months vs 18 months in patients who did not receive taxanes). The incorporation of both anthracylines and taxanes into the pre-operative regimen is now considered standard of care for women with IBC.
An important component of the use of pre-operative chemotherapy is the assessment of response to treatment, with pCR considered to be an intermediate surrogate marker for improved survival.[38] The question of whether pCR is a prognostic marker in IBC has also been investigated. Hennessy et al[39] demonstrated that among women with IBC who had cytologically confirmed axillary lymph node metastases, 5-year recurrence-free and overall survival rates in the group of patients who attained pCR in the axillary lymph nodes (82.5% and 78.6%, respectively) were higher than the rates in those who had evidence of residual disease (37.1% and 25.4%, respectively). Ueno et al[35] reported 15-year survival rates in women with IBC receiving pre-operative chemotherapy of 44%, 31%, and 7% in those patients who achieved a complete response, a partial response, and a less than partial response, respectively. Thus, from the available data it appears that among women with IBC, response to pre-operative chemotherapy plays a similar prognostic role to that observed among women with non-IBC.
Novel targeted agents
TABLE
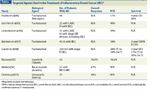
Targeted Agents Used in the Treatment of Inflammatory Breast Cancer (IBC)
Our deeper understanding of the molecular biology of IBC has led to the identification of several prime molecular targets that may help with the development of therapeutic agents, with the goal of further improving prognostic outcomes (Table). The overexpression and/or gene amplification of HER2 is of particular interest for several reasons. First, as described earlier, there appears to be an increased frequency of HER2 positivity in IBC tumors,[16] which makes HER2 an important target. Second, prospective clinical trials in women with non-IBC tumors have demonstrated that the incorporation of trastuzumab (Herceptin; a humanized monoclonal antibody that targets the HER2 protein) into chemotherapy regimens has not only resulted in a significant improvement in survival among women with HER2-positive early[40,41] and advanced-stage[42] breast cancer, but has also been shown to increase pCR rates in these same populations when incorporated pre-operatively.[43] The efficacy of trastuzumab in IBC has also been investigated.[44-49]
Gianni et al[48] recently reported on the results of a prospective phase III randomized clinical trial that looked at the incorporation of one year of pre-operative and adjuvant trastuzumab into the treatment of 235 women with LABC, of whom 63 had IBC. All women received an anthraycline- and taxane-based pre-operative regimen. The authors reported a 3-year event-free survival of 71% in the women who received trastuzumab and of 56% in those who did not. The pCR rate was also reported to be higher in the women who received trastuzumab than in those who did not (38% vs 19%). In a recent small retrospective study, Dawood et al[49] reported on a cohort of patients with HER2-positive IBC who had received pre-operative trastuzumab. The authors reported a pCR rate of 62.5%, with 37.5% of patients achieving a partial response. From the data described, it appears reasonable to assume that trastuzumab improves prognostic outcomes and increases pCR rates among women with HER2-positive IBC-and to conclude that trastuzumab should be considered an essential component of treatment in this subset of patients.
The efficacy of lapatinib (Tykerb), a reversible tyrosine kinase inhibitor of HER1 (ErbB1) and HER2 (ErbB2) tyrosine kinases, is also being investigated in women with IBC.[50-53] Results from a phase II study of 42 women with HER2-positive IBC who were treated with pre-operative lapatinib and paclitaxel included an overall clinical response rate of 78.6% and a pCR rate of 18.2%.[53] Lapatinib-based combinations are currently being investigated in larger prospective cohorts of patients with IBC.
VEGF (vascular endothelial growth factor) receptor has also been shown to be highly expressed in IBC,[19] which makes it another potential therapeutic target. Bevacizumab (Avastin), a recombinant humanized monocolonal antibody that binds to VEGF, has been investigated in combination with chemotherapy and has been shown to improve progression-free survival in women with metastatic breast cancer.[54,55] This agent is currently being investigated in women with IBC. Wedam et al[56] reported on a pilot study of 21 patients with untreated IBC or LABC who received 1 cycle of pre-operative bevacizumab followed by 6 cycles of pre-operative bevacizumab in combination with doxorubicin (Adriamycin) and docetaxel (Taxotere). The investigators reported an overall response rate of 67% (95% CI = 43%–85.4%), with one patient attaining a pCR. Semaxanib (SU5416), a small molecular that inhibits VEGF-mediated signaling through the FLK-1 and KDR tyrosine kinase receptor, is also being investigated in IBC. In a phase IB study, Overmoyer et al[57] reported on a group of 21 patients with stage III and IV IBC who received a pre-operative combination of SU5416 and doxorubicin. The investigators reported an overall response rate of 90%. Six patients (32%) achieved a complete clinical response, and 13 (68%) achieved a clinical partial response. At a median follow-up of 50 months, the median event-free survival was reported as 31 months (range, 7 to 54 months). The investigators noted that neutropenia was a dose-limiting toxicity and that congestive cardiac failure occurred in 22% of patients.
Other targets that are currently being investigated in IBC include the WNT1-inducible signaling pathway protein 3, epidermal growth factor receptor (EGFR), p27Kip1, and the Ras homolog gene family member C guanosine triphosphatase.[58]
Advances in surgery
Following the administration of pre-operative chemotherapy, treatment is targeted toward local control. The current standard of care following an adequate clinical response to pre-operative chemotherapy is a modified radical mastectomy of the affected breast. Before the introduction of pre-operative chemotherapy, a modified radical mastectomy was technically not possible in the majority of cases due to the extent of the disease on the chest wall, which made it difficult to achieve negative margins. Pre-operative chemotherapy made surgery a technically feasible option. Several facts highlight the important role of definitive surgery in IBC: first, the fact that although nearly 70% of patients with IBC present with locoregional disease, only a small percentage have evidence of distant metastases at diagnosis,[10] and second, the fact that in approximately 60% of cases, data indicate that physical examination and imaging modalities can underestimate the true extent of residual disease in the affected breast parenchyma and its overlying skin following pre-operative treatment.[59] Retrospective data also confirm the effectiveness of mastectomy, which has a positive impact on local and distant recurrence rates in women with IBC.[60] The role of sentinel lymph node biopsy has also been investigated in women with IBC.[61] However, the unacceptably high rate of inaccuracy of sentinel lymph node biopsy in women with IBC precludes its use in this setting at this time.
Advances in radiation therapy
Post-mastectomy radiation therapy is the standard of care to further improve local control.[62] Research has focused on using accelerated-hyperfractionated radiation therapy in the treatment of women with IBC in order to prevent rapid repopulation of IBC tumor cells between treatments, which has been hypothesized to be a mechanism of radiation therapy resistance. In 2008, Bristol et al[63] reported on a cohort of 192 women with nonmetastatic IBC who were able to complete a planned course of pre-operative chemotherapy, undergo a modified radical mastectomy, and receive post-mastectomy radiation therapy at the M.D. Anderson Cancer Center. Most often, post-mastectomy radiation therapy was delivered in a dose-dense, twice-daily fractionation to 66 Gy. The investigators reported that in this population, the 5-year actuarial locoregional control was 84%, the distant metastasis-free survival was 47%, and the overall survival was 51%. The authors further noted that the patients that appeared to benefit the most from the escalation of the post-mastectomy radiation dose to 66 Gy were those who responded poorly to chemotherapy, those with positive margins, and those younger than 45 years.
Impact of Advances on Survival Outcomes: What Progress Have We Made?
When looking at the survival outcomes of women with IBC, several questions need to be addressed. First, has the overall survival of women diagnosed with IBC improved? The answer to this question is a clear-cut yes. With the introduction of enhanced diagnostic techniques and sequential treatment with pre-operative chemotherapy, surgery, and radiation therapy, median overall survival has significantly improved from approximately 15 months to 40 months;[3, 35] studies document that approximately 28% of women with IBC are alive and free of disease at 15 years.[35] Second, has there been a steady improvement in the survival of women with IBC over the past three decades? The answer to this question is not clear cut. Gonzalez-Angulo et al[64] looked at whether the survival of women with IBC who were treated at the M.D. Anderson Cancer Center had improved over the past 30 years. The authors reported that in the multivariable models, after adjusting for a number of patient and tumor characteristics, increasing year of diagnosis was not associated with a decrease in either the risk of recurrence (hazard ratio [HR] =1.00; 95% CI = 0.97–1.04) or death (HR = 0.97; 95% CI = 0.94–1.01). However, the authors of the study acknowledged a number of limitations that could have contributed to the observed results. Third, is the survival of women with IBC in the 21st century similar to that of women with LABC? In a retrospective review of women in the Surveillance, Epidemiology and End Results (SEER) registry, our group has recently shown that women with IBC who were treated between 2004 and 2007 in settings where a multidisciplinary disciplinary approach to treatment is considered standard of care, continue to have poorer survival outcomes when compared with women with non-IBC LABC (personal communication with authors; paper in press).
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Bevacizumab (Avastin)
Docetaxel (Taxotere)
Doxorubicin (Adriamycin)
Lapatinib (Tykerb)
Paclitaxel
Semaxanib (SU5416)
Trastuzumab (Herceptin)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Introducing the First Consensus Guidelines
It is clear that important and significant progress has been made in the field of IBC. However, there is still a long way to go. Despite an improved treatment approach that has had a positive impact on the prognostic outcome of this disease, the survival outcomes are still poor and still inferior to those of non-IBC LABC. The future of IBC will depend on a deeper understanding of the disease at the molecular level. The data available so far indicate that molecular subtypes defined within non-IBC tumors also exist within IBC tumors. Future research will need to concentrate on defining the prognostic and predictive value of these subtypes in IBC as well as identifying potential therapeutic targets.
One of the biggest challenges facing researchers in the field of IBC is the rarity of the disease; its rarity has an impact on both epidemiological and translational research. To address this issue, a group of international experts in December 2008 formed the first IBC consortium. This consortium formulated and recently published the first international guidelines on the diagnosis and management of IBC, with the objective of standardization.[65] With the standardization of diagnostic criteria and therapeutic strategies in IBC, and with the consortium encouraging collaboration of research at both the clinical and molecular level, the next decade is bound to see further progress in the field of IBC.
Financial Disclosure:Dr. Dawood has received honoraria from Roche. Dr. Cristofanilli has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Breast. In: Green FL, Page DL, Fleming ID, et al, editors. AAJCC cancer staging manual, 6th ed. New York: Springer-Verlag: 2002. p. 225-81
2. Levine PH, Steinhorn SC, Ries LG, Aron JL. Inflammatory breast cancer: the experience of the Surveillance, Epidemiology, and End Results (SEER) program. J Natl Cancer Inst. 1985;74:291-7.
3. Bozzetti F, Saccozzi R, De Lena M, et al. Inflammatory cancer of the breast: analysis of 114 cases. J Surg Oncol. 1981;18:355-61.
4. Barker JL, Nelson AJ, Montague ED. Inflammatory carcinoma of the breast. Radiology. 1976;121:173-6.
5. Zucali R, Uslenghi C, Kenda R, et al. Natural history and survival of inoperable breast cancer treated with radiotherapy and radiotherapy followed by radical mastectomy. Cancer. 1976;37:1422-31.
6. Low J, Berman A, Steinber S, et al. Long term follow up for locally advanced and inflammatory breast cancer patients treated with multimodality therapy. J Clin Oncol. 2004;22:4065-74.
7. Ueno NT, Buzdar AU, Singletary SE, et al. Combined modality treatment of inflammatory breast carcinoma: twenty years of experience at M.D. Anderson Center. Cancer Chemother Pharmacol. 1997;40:321-329
8. Bertucci F, Finetti P, Cervera N, et al. Gene expression profiling and clinical outcome in breast cancer. OMICS. 2006;10:429-43.
9. Mourali N, Muenz LR, Tabbane F, et al. Epidemiologic features of rapidly progressing breast cancer in Tunisia. Cancer. 1980;46:2741-6.
10. Wingo PA, Jamison PM, Young JL, Gargiullo P. Population-based statistics for women diagnosed with inflammatory breast cancer (United States). Cancer Causes Control. 2004;15:321-8.
11. Chang S, Buzdar AU, Hursting SD. Inflammatory breast cancer and body mass index. J Clin Oncol. 1998;16:3731-5.
12. Pogo BG, Holland JF, Levine PH. Human mammary tumor virus in inflammatory breast cancer. Cancer. 2010;116(11 Suppl):2741-4.
13. Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: A review. J Clin Oncol. 1992;10:1014-24.
14. Gruber G, Ciriolo M, Altermatt HJ, et al. Prognosis of dermal lymphatic invasion with or without clinical signs of inflammatory breast cancer. Int J Cancer. 2004;109:144-8.
15. Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res. 2000;2:423-29.
16. Turpin E, Bieche I, Berthaeau P, et al. The increased incidence of ERBB2 over expression and TP53 mutation in inflammatory breast cancer. Oncogene. 2002;21:7593-7.
17. Kleer CG, van Golen KL, Braun T, et al. Persistent E-cadherin expression in inflammatory breast cancer. Mod Pathol. 2002;14:458-64.
18. van Golen KL, Davies S, Wu ZF, et al. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511-9.
19. Van der Auwera I, Van Laere SJ, Van Den Eynden GG, et al. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res. 2004;10:7965-71.
20. Cabioglu N, Gong Y, Islam R, et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol. 2007;18:1021-9.
21. Bertucci F, Finetti P, Rougemont J, et al. Gene expression profiling for molecular characterization of inflammatory breast cancer and prediction of response to chemotherapy. Cancer Res. 2004; 64:8558-65.
22. Bertucci F, Finetti P, Rougemont J, et al. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res. 2005;65:2170-8.
23. Van Laere S, Van der Auwera I, Van den Eynden GG, et al. Distinct molecular signature of inflammatory breast cancer by cDNA microarray analysis. Breast Cancer Res Treat. 2005;93:237-46.
24. Van Laere SJ, Van den Eynden GG, Van der Auwera I, et al. Identification of cell-of-origin breast tumor subtypes in inflammatory breast cancer by gene expression profiling. Breast Cancer Res Treat. 2006;95:243-55.
25. Van Laere S, Van der Auwera I, Van den Eynden G, et al. Distinct molecular phenotype of inflammatory breast cancer compared to non-inflammatory breast cancer using Affymetrix-based genome-wide gene-expression analysis. Br J Cancer. 2007;97:1165-74.
26. Bièche I, Lerebours F, Tozlu S, et al. Molecular profiling of inflammatory breast cancer: identification of a poor-prognosis gene expression signature. Clin Cancer Res. 2004;10:6789-95.
27. Dressman HK, Hans C, Bild A, et al. Gene expression profiles of multiple breast cancer phenotypes and response to neoadjuvant chemotherapy. Clin Cancer Res. 2006;12(3 Pt 1):819-26.
28. Nguyen DM, Sam K, Tsimelzon A, et al. Molecular heterogeneity of inflammatory breast cancer: a hyperproliferative phenotype. Clin Cancer Res. 2006;12:5047-54.
29. Boersma BJ, Reimers M, Yi M, et al. A stromal gene signature associated with inflammatory breast cancer. Int J Cancer. 2008;122:1324-32.
30. Van Laere S, Beissbarth T, Van der Auwera I, et al. Relapse-free survival in breast cancer patients is associated with a gene expression signature characteristic for inflammatory breast cancer. Clin Cancer Res. 2008;14:7452-60.
31. Yang WT. Advances in imaging of inflammatory breast cancer. Cancer. 2010;116(11 Suppl):2755-7.
32. Yang WT, Le-Petross HT, Macapinlac H, et al. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat. 2008;109:417-26.
33. Carkaci S, Macapinlac HA, et al. Retrospective study of 18F-FDG PET/CT in the diagnosis of inflammatory breast cancer: preliminary data. J Nucl Med. 2009;50:231-8.
34. Early Breast Cancer Trialists’ Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687-1717.
35. Ueno NT, Buzdar AU, Singeltary SE, et al. Combined modality treatment of inflammatory breast carcinoma: twenty years of experience at M.D Anderson Center. Cancer Chemother Pharmacol. 1997;40:321-9.
36. Baldini E, Gardin G, Evangelista G, et al. Long-term results of combined-modality therapy for inflammatory breast cancer. Clin Breast Cancer. 2004;5:358-63.
37. Cristofanilli M, Gonzalez-Angulo AM, Buzdar AU, et al. Paclitaxel improves the prognosis in estrogen receptor negative inflammatory breast cancer: the M.D Anderson Cancer Center experience. Clin Breast Cancer. 2004;4:415-9.
38. Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathological primary tumor axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460-9.
39. Hennessy BT, Gonzalez-Angulo AM, Hortobagyi GN, et al. Disease-free and overall survival after pathological complete disease remission of cytologically proven inflammatory breast carcinoma axillary lymph node metastases after primary systemic chemotherapy. Cancer. 2006;106:1000-6.
40. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-72.
41. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-84.
42. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-92.
43. Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676-85.
44. Hurley J, Doliny P, Reis I, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-postive locally advanced breast cancer. J Clin Oncol. 2006;24:1831-8.
45. Van Pelt AE, Mohsin S, Elledge RM, et al. Neoadjuvant trastuzumab and docetaxel in breast cancer: preliminary results. Clin Breast Cancer. 2003;4:348-353.
46. Limentani SA, Brufsky AM, Erban JK, et al. Phase II study of neoadjuvant docetaxel, vinorelbine, and trastuzumab followed by surgery and adjuvant doxorubicin plus cyclophosphamide in women with human epidermal growth factor receptor 2-overexpressing locally advanced breast cancer. J Clin Oncol. 2007;25:1232-8.
47. Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol. 2003;21:46-53 .
48. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377-84.
49. Dawood S, Gong Y, Broglio K, et al. Trastuzumab in primary inflammatory breast cancer (IBC): high pathological response rates and improved outcome. Breast J. 2010. [Epub ahead of print]
50. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733-43.
51. Burris HA 3rd, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305-13.
52. Spector NL, Blackwell K, Hurley J, et al. EGF103009, a phase II trial of lapatinib monotherapy in patients with relapsed/refractory inflammatory breast cancer (IBC): clinical activity and biologic predictors of response. J Clin Oncol. ASCO Annual Meeting Proceedings 2006. Part I. Vol 24. No. 18S (June 20 Supplement); 502.
53. Boussen H, Cristofanilli M, Zaks T, et al. Phase II study to evaluate the efficacy and safety of neoadjuvant lapatinib plus paclitaxel in patients with inflammatory breast cancer. J Clin Oncol. 2010;28:3248-55.
54. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-76.
55. Alvarez RH, Valero V, Hortobagyi GN. Emerging targeted therapies for breast cancer. J Clin Oncol. 2010;28:3366-79.
56. Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769-77.
57. Overmoyer B, Fu P, Hoppel C, et al. Inflammatory breast cancer as a model disease to study tumor angiogenesis: results of a phase IB trial of combination SU5416 and doxorubicin. Clin Cancer Res. 2007;13:5862-8.
58. Yamauchi H, Ueno NT. Targeted therapy in inflammatory breast cancer. Cancer. 2010;116(11 Suppl):2758-9.
59. Vlastos G, Fornage BD, Mirza NQ, et al. The correlation of axillary ultrasonography with histologic breast cancer downstaging after induction chemotherapy. Am J Surg. 2000;179:446-52.
60. Flemming RY, Asmar L, Buzdar AU, et al. Effectiveness of mastectomy by response to induction chemotherapy for control in inflammatory breast carcinoma. Ann Surg Oncol. 1997;4:452-61.
61. Stearns V, Ewing CA, Slack R, et al. Sentinel lymphadenopathy after neoadjuvant chemotherapy for breast cancer may reliably represent the axilla except for inflammatory breast cancer. Ann Surg Oncol. 2002;9:235-42.
62. Woodward WA, Buchholz TA. The role of locoregional therapy in inflammatory breast cancer. Semin Oncol. 2008;35:78-86.
63. Bristol IJ, Woodward WA, Strom EA, et al. Locoregional treatment outcomes after multimodality management of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2008;72:474-84.
64. Gonzalez-Angulo AM, Hennessy BT, Broglio K, et al. Trends for inflammatory breast cancer: is survival improving? Oncologist. 2007;12:904-12.
65. Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2010 Aug 9. [Epub ahead of print]