Implications of Mutation Profiling in Myeloid Malignancies-PART 1: Myelodysplastic Syndromes and Acute Myeloid Leukemia
In this first part of our two-part review, we introduce mutation profiling as a relevant clinical tool for hematologists treating patients with myeloid malignancies.
Oncology (Williston Park). 32(4):e38-e44.

Figure 1. Genes Recurrently Mutated in Myeloid Malignancies Categorized by Oncogenic Mechanism
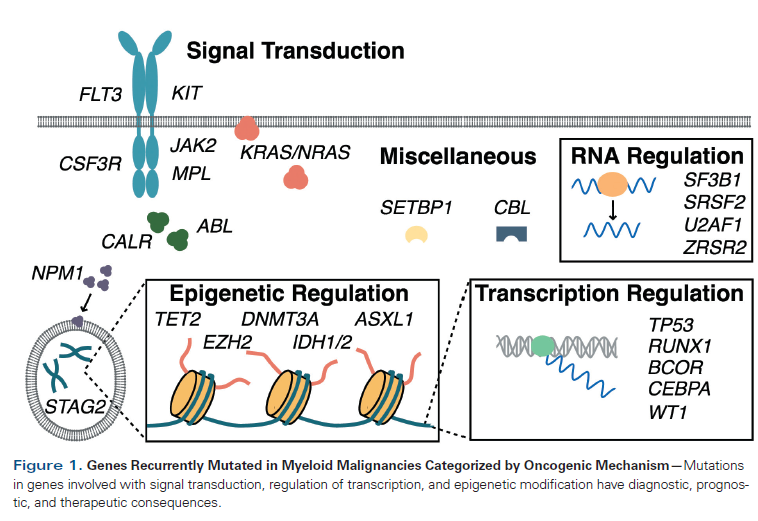
Table 1. Revised International Prognostic Scoring System (IPSS-R) for Myelodysplastic Syndrome
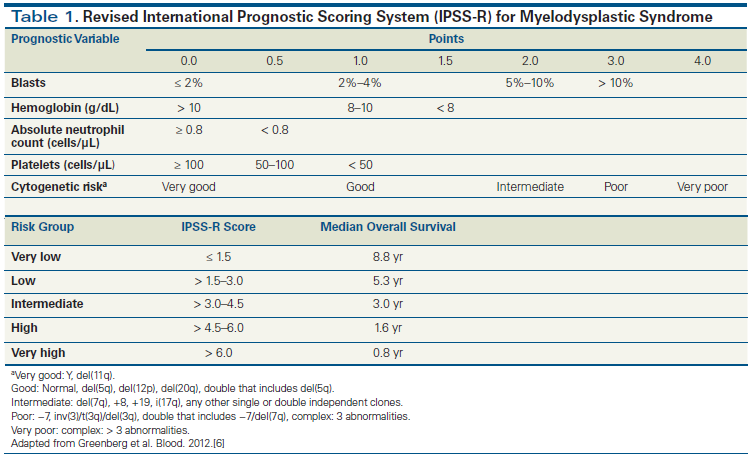
Table 2. Prevalence of Genes Mutated by Disease Type and Stratified by Cellular Function
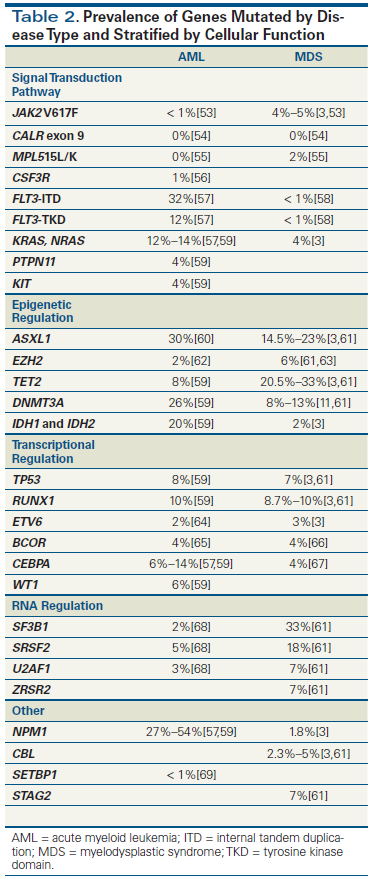
Table 3. Prognostic Implications of Mutations in Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS)
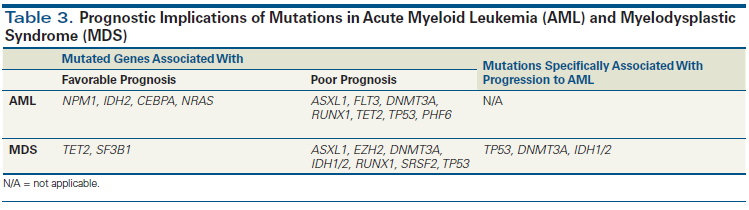
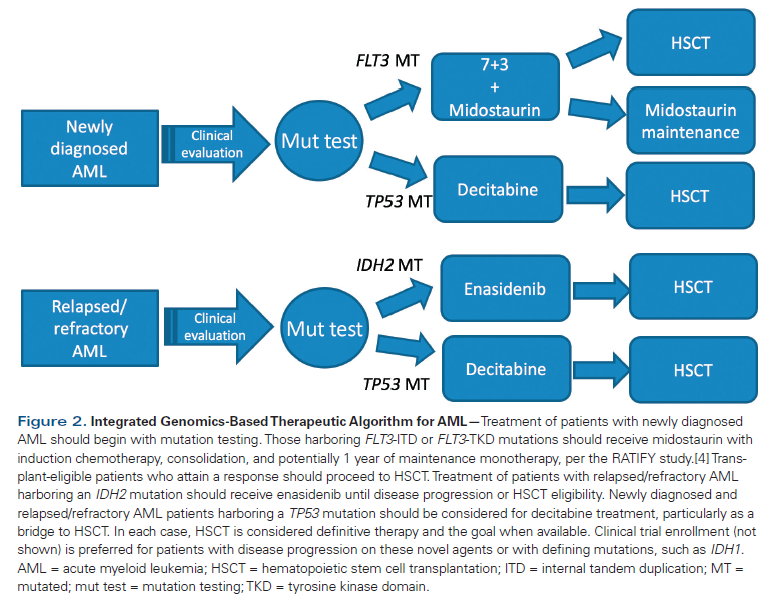
Table 4. Cytogenetic Risk Classification for Acute Myeloid Leukemia
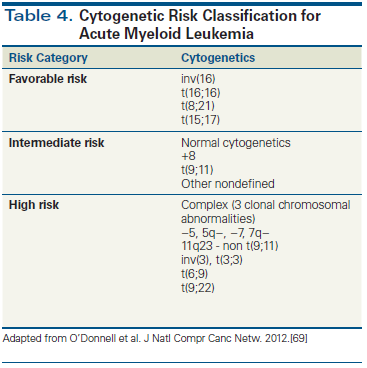
Table 5. Molecular Targets for Acute Myeloid Leukemia
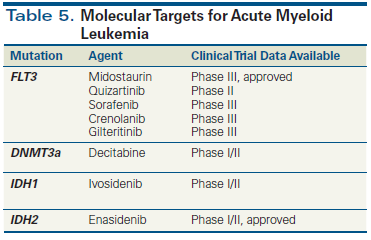
Figure 2. Integrated Genomics-Based Therapeutic Algorithm for AML
The advent of high-throughput gene sequencing has revolutionized our understanding of the genetic mutations that drive myeloid malignancies. While these mutations are of interest pathobiologically, they are increasingly being recognized as clinically meaningful in providing diagnostic, prognostic, and therapeutic information to guide patient care. In this first part of our two-part review, we introduce mutation profiling as a relevant clinical tool for hematologists treating patients with myeloid malignancies. Next, we discuss the diagnostic and prognostic role of mutation profiling in myelodysplastic syndrome and acute myeloid leukemia. Finally, we detail the therapeutic implications of specific mutations in myelodysplastic syndrome and acute myeloid leukemia. In Part 2, we will discuss similar clinical approaches using mutation profiling in myeloproliferative neoplasms and other myeloid malignancies.
Introduction
Enormous strides have been made in recent years that have furthered our understanding of the underlying genetic alterations driving myeloid malignancies. High-throughput gene sequencing, the technological innovation behind these advances, has resulted in the identification of numerous recurrent somatic pathogenetic mutations. Genes that are frequently mutated in myeloid malignancies possess a multitude of cellular functions that can be generally grouped into those that regulate 1) RNA, 2) the transcriptome, and 3) the epigenome (Figure 1). With a growing appreciation of the biological consequences of these mutations, there is also heightened recognition of their clinical implications in the management of patients with myeloid malignancies such as myelodysplastic syndrome (MDS), acute myeloid leukemia (AML), myeloproliferative neoplasms, and other related myeloid neoplasms. Molecular abnormalities identified in the blood and bone marrow cells of patients with myeloid malignancies can provide diagnostic[1] and prognostic clues[2,3] and therapeutic direction to the clinician.[4] Therefore, understanding the clinical relevance of molecular testing holds the potential to greatly personalize the care of these patients. Despite the routine incorporation of mutation profiling in the clinic, questions remain regarding its optimal use in the care of an individual patient with a myeloid malignancy. By describing the current landscape of relevant genomic alterations in myeloid malignancies, we hope to provide guidance and clarity to clinicians struggling to understand the clinical ramifications of mutation profiling for their patients.
MDS: Prognostic Implications
The International Prognostic Scoring System (IPSS) has been used as a prognostic tool to guide therapeutic decision making based on inherent risk associated with MDS. It incorporates conventional cytogenetics, bone marrow blast percentage, and peripheral blood count parameters.[5] More recently, the IPSS has been revised (and validated) to include updated cytogenetic information and more detail on depth of cytopenias and degree of blast percentage (Table 1).[6] None of the current risk stratification tools incorporates genomic data.[7,8] However, our understanding of the molecular pathogenesis of MDS has grown over the past decade with the identification of recurrent mutation events. The most common mutated genes in MDS include those involved in DNA methylation (TET2, DNMT3A, and IDH1/2), chromatin modification (ASXL1, EZH2), transcriptional regulation (RUNX1, TP53), signal transduction (NRAS, KRAS), and RNA splicing (SF3B1, SRSF2) (Table 2).[9]
Information regarding mutations can also have prognostic significance in MDS (Table 3). For example, mutations involving TET2 have been shown to be associated with a favorable prognosis in some studies, although they were neutral in others. MDS patients harboring TET2 mutations had a longer overall survival (OS) than patients without such mutations, independent of age, IPSS score, and transfusion requirements. Additionally, the absence of TET2 mutations was associated with a fourfold increased risk of death.[10] In contrast, a more recent and larger study did not identify a significant OS difference in MDS patients with monoallelic or biallelic TET2 mutations vs those without such mutations.[3]
DNMT3A is an epigenetic modulator that encodes for DNA methyltransferase 3 alpha. An investigation of 150 de novo MDS cases demonstrated that those patients with DNMT3A mutations had worse OS and more rapid evolution to AML.[11] However, this prognostic value may vary based on the presence of concurrent mutations and the IPSS risk group. For example, mutated SF3B1 co-occurring with mutated DNMT3A may mitigate the negative effect of DNMT3A mutations on survival in a lower-risk population (4.16 years vs 1.45 years; P = .35).[12]
Similar to TET2 and DNMT3A, IDH1/2 mutations lead to global DNA hypermethylation. Mutations involving IDH1/2 are associated with a shorter OS and a higher rate of transformation to AML.[13] Recent analysis of data from 3,000 MDS patients found that IDH2 mutations were associated with shorter OS. In contrast, the association between IDH1 mutations and shorter OS did not reach statistical significance, highlighting the variation in impact of mutations occurring in similar genes.[14] Additionally, a study of patients with MDS or secondary AML who received hematopoietic stem cell transplantation (HSCT) suggested that the presence of mutated IDH2 after HSCT may be associated with reduced OS (hazard ratio, 2.6).[15] This negative prognostic impact has not been observed for mutated IDH1.
ASXL1 and EZH2 are epigenetic modulators that act through histone modification and are significant predictors of poor OS after adjustment for IPSS risk group.[3] RUNX1 has also been studied in MDS patients. Those with mutated RUNX1 had higher neutrophil counts and higher frequency of -7/7q deletion than those with wild-type RUNX1, and the presence of the mutant allele was closely associated with a shorter OS.[16,17]
TP53 mutation has been shown to be more predictive of OS than IPSS score.[18] Patients with MDS harboring a TP53 mutation had shorter leukemia-free survival than those with wild-type status. Moreover, patients with normal karyotype and TP53 mutations survived for a similarly short period (median, 9.8 months) compared with patients who had a complex karyotype and TP53 mutations (median, 7.6 months).[19] In a retrospective study of the outcome after HSCT in 87 MDS patients, the presence of TP53, TET2, and DNMT3A before transplant were all independently associated with reduced OS after transplant.[20] SF3B1 and SRSF2 encode components of the nuclear ribonucleoprotein integral to the spliceosome.[21] SF3B1 mutations are present in over 20% of MDS patients and more specifically in 81% of those with refractory anemia with ring sideroblasts (RARS) or refractory cytopenia with multilineage dysplasia and ring sideroblasts.[9,22] Patients with SF3B1 mutations have a significantly better OS and a reduced incidence of disease progression than patients lacking them.[22] SRSF2 mutations, detected in 12% to 15% of MDS patients, are associated with an inferior OS, especially in the lower-risk IPSS group; this may be attributed to the close association of SRSF2 mutations with advanced age.[23,24]
AML: Prognostic Implications
Cytogenetic-based risk stratification remains the mainstay of risk-adapted treatment strategies in AML (Table 4). Numerous studies have examined the clinical impact of mutated NPM1, FLT3, and IDH1/2 in AML. The presence of mutated NPM1 is associated with a higher rate of complete remission after induction chemotherapy.[25] When mutated NPM1 coexists with wild-type FLT3, it is an independent predictor of favorable OS and event-free survival. Additionally, when NPM1 is mutated along with IDH1/2, it is associated with improved 3-year survival in intermediate-risk (by karyotype) AML compared with mutated NPM1 with wild-type IDH1/2.[26]
The presence of NPM1 transcripts can also signal submicroscopic minimal residual disease. This was demonstrated in a recent study of 346 patients with NPM1-mutated AML in which reverse-transcriptase quantitative polymerase chain reaction was used to detect residual NPM1 transcripts. Minimal residual disease at first complete response (CR) was detected in 16% of patients and associated with a higher risk for relapse at 3 years (82% vs 30%) and lower rate of survival (24% vs 75%). It was also an independent predictor of death in a multivariate analysis.[27]
FLT3 mutation status is an important molecular variable to consider in AML patients classified as intermediate risk by cytogenetic-based risk stratification. Overall, the FLT3 internal tandem duplication (FLT3-ITD) mutation is associated with an unfavorable prognosis and is useful to further segregate higher-risk patients who may benefit from more aggressive therapy.[28] FLT3-ITD has also been shown to be predictive of poor response to induction chemotherapy and HSCT.[29]
A meta-analysis of 8,121 patients demonstrated that patients with mutations in IDH1 have an inferior OS when compared with patients who do not have this mutation.[30] Mutated IDH2, interestingly, is associated with improved survival and decreased rate of relapse; however, when it occurs in conjunction with FLT3-ITD, this survival benefit is negated.[26]
DNMT3A is a commonly mutated gene in AML, clustering within the intermediate-risk cytogenetic category, and overall is associated with inferior survival.[31] However, this relationship is also affected by coexisting mutations. In high-risk patients with FLT3-ITD and NPM1 mutations, DNMT3A mutations are associated with decreased OS, relapse-free survival, and CR.
Mutated TET2 occurs in approximately 13% of AML patients with intermediate-risk cytogenetics and is associated with a poor prognosis in them, especially when coexisting with FLT3-ITD, NPM1 wild-type, or other unfavorable genotypes.[32] A recent analysis of patients with normal-karyotype AML undergoing induction chemotherapy demonstrated that a homozygous TET2 mutation (approximately 25% of mutated TET2 cases) is an independent predictor of relapse.[33]
Mutated CEBPA is considered a good prognostic marker in AML. In a multivariate analysis, biallelic mutant CEBPA was associated with a favorable prognosis and longer disease-free survival. In contrast, a single CEBPA mutation showed no difference in outcome compared with wild-type CEBPA.[34] This highlights the heterogeneity even among AML patients harboring mutated CEBPA.
Several other mutations also have prognostic implications in patients with AML. Mutated RUNX1, for instance, is associated with lower CR rates after induction chemotherapy and shorter disease-free survival and OS in de novo AML.[35] Mutated RUNX1 is strongly associated with adverse prognosis in normal-karyotype AML.[36] TP53 mutations are associated with abnormal karyotype, older age, and worse OS.[37] The presence of mutated c-KIT predicts a higher relapse risk in inv(16) and t(8;21) AML.[38] Mutations involving PHF6 are associated with reduced OS in patients younger than 60 years of age.[26] Finally, mutated ASXL1 is the most frequent mutation in intermediate-risk karyotype AML and is an independent adverse variable for OS.[39,40]
Mutation analysis clearly offers valuable prognostic information to clinicians treating patients with AML, albeit at the expense of increasing complexity. These mutations must be examined collectively, as clinical implications vary when they occur concurrently. This was convincingly demonstrated in a recent study of 1,540 patients with AML, which proposed a classification for genomic subgroups. A total of 5,234 driver mutations were identified involving 76 genes, and 1 or more driver mutations were identified in 86% of cases. Distinct patterns of co-mutation were shown to exist, and a total of 11 subgroups were defined within 3 categories (chromatin splicesome, TP53-aneuploidy, and IDH2 R172). Furthermore, some subgroups, particularly NPM1-mutated AML (the largest subgroup, at 27%), had patterns of co-mutation that could either positively or negatively impact prognosis.[41]
AML and MDS: Treatment Implications
Current curative-intent treatments for AML rely heavily on standard cytotoxic induction and consolidation chemotherapy with and without HSCT. However, as the genetics of AML have become increasingly elucidated, the pace of clinical development of targeted therapies has become rapid, contributing to the approval of two novel agents in 2017 by the US Food and Drug Administration (Table 5). Mutation profiling can now be incorporated into therapeutic decision making in patients with both newly diagnosed and relapsed/refractory disease (Figure 2).
One of the most promising AML-associated targets is FLT3. Sorafenib, quizartinib, gilteritinib, and crenolanib have all been tested in FLT3-mutated AML in the newly diagnosed and relapsed/refractory settings, with generally encouraging results.[42] Midostaurin is the sole FLT3 inhibitor approved for use in patients with AML. Recently, RATIFY, a multicenter, international, phase III trial, randomized 717 previously untreated patients with FLT3-mutated AML to daunorubicin/cytarabine induction and cytarabine-based consolidation in combination with midostaurin or placebo, followed by 1 year of maintenance therapy. There was a significant improvement in OS, with a 23% reduced mortality risk in the midostaurin-treated group. Additionally, there was significant improvement in event-free survival with midostaurin treatment that was evident even when the data were censored for patients who received HSCT.[4] These results establish a benefit of incorporating midostaurin in induction therapy for patients harboring an FLT3 mutation. An ongoing trial of midostaurin maintenance after HSCT (ClinicalTrials.gov identifier: NCT01883362) should shed light on its optimal use in those receiving transplant. Currently, midostaurin is indicated for use at a dosage of 50 mg twice daily by mouth (days 8 to 21) with cytarabine and daunorubicin induction therapy and with cytarabine consolidation and maintenance monotherapy in patients with newly diagnosed FLT3 mutation-positive AML.
Other novel targeted therapies that are being actively investigated include enasidenib, a potent reversible inhibitor of mutant IDH2. Enasidenib is the first agent approved for the treatment of patients with relapsed/refractory AML harboring an IDH2 mutation; its approval was based on the results of a phase I/II clinical trial of 199 patients (ClinicalTrials.gov identifier: NCT01915498).[43] After a median follow-up of 6.6 months, 23% of patients attained CR/CR with incomplete blood count recovery (CRi) lasting a median of 8.2 months. The median time to CR/CRi was 3.7 months. Differentiation syndrome, which requires prompt recognition and intervention, was reported at a frequency of 14%. Enasidenib is currently approved as monotherapy for patients with relapsed/refractory AML harboring an IDH2 mutation at a dose of 100 mg daily by mouth until disease progression.
Additionally, an ongoing phase I clinical trial is studying the use of ivosidenib, a selective inhibitor of IDH1 mutations (ClinicalTrials.gov identifier: NCT02074839). Preliminary data show that 7 of 14 efficacy-evaluable subjects demonstrated objective responses, including 4 with CR (28%) and 2 (14%) with CRi; durable responses of up to 5.7 months were noted.[44]
Another prognostically relevant mutation, DNMT3A, is associated with worse overall prognosis in AML patients. However, a study of 46 patients with normal-karyotype AML treated with decitabine demonstrated higher rates of CR and a trend toward longer OS in patients with a DNMT3A mutation, suggesting that these patients are more sensitive to this hypomethylating agent.[45] Also, patients with DNMT3A-mutated AML may have better survival with dose attenuation of daunorubicin within the induction regimen, as seen in a retrospective analysis of 152 patients with de novo AML.[46]
The implications of mutations on the timing of and/or indication for HSCT in AML have yet to be fully elucidated. FLT3-ITD is associated with worse outcomes after HSCT.[47] In one study comparing chemotherapy consolidation with HSCT during first CR in patients with FLT3-ITD-mutated AML, decreased risk of relapse was seen in the HSCT group, although there was no significant difference in OS. A landmark analysis of 5-year relapse-free survival demonstrated benefit of HSCT in 437 patients with FLT3-ITD AML. However, the benefit appeared to disappear if HSCT was delayed, suggesting that transplantation soon after induction may be superior to consolidation with chemotherapy.[48] Another retrospective study of 133 patients with newly diagnosed AML demonstrated similar OS between FLT3-ITD and FLT3 wild-type patients, but with a shorter relapse-free survival in FLT3-ITD patients who did not undergo HSCT than in patients who did.[49] Few data exist for the other common mutations in AML; however, a subgroup analysis showed favorable impact on relapse-free survival in patients with a RUNX1 mutation who underwent HSCT.[50]
While numerous mutations have been characterized in patients with MDS, targeted therapies are still not commercially available to clinicians. Mutations involving TET2 and DNMT3A may serve as predictive biomarkers for therapeutic response to DNMT inhibitor therapy with azacytidine or decitabine and therefore guide treatment decisions.[51,52] In a retrospective study of 92 MDS patients, mutated TET2 and/or DNMT3A retained significance as an independent predictor for improved progression-free response with DNMT inhibitor therapy.[51] In a cohort of 213 MDS patients treated with DNMT inhibitor therapy, the response rate was superior in TET2-mutated patients and particularly evident in the subgroup lacking a concurrent ASXL1 mutation.[52]
Only limited retrospective studies have evaluated the role of mutations in outcomes after HSCT in patients with MDS. In one study of 87 patients, median post-HSCT OS was significantly shorter in patients carrying mutations in TP53, TET2, or DNMT3A.[20]
Conclusion
Mutation profiling for patients with MDS and AML is commercially available and widely included in the evaluation and management of many patients in the community. The evolving prognostic implications of integrated genomics in the care of these patients will continue to refine our decision making as it relates to the use of currently available therapies, such as hypomethylating agents vs HSCT in MDS patients. Further, it now clearly defines subpopulations of AML patients deserving of targeted therapies, such as those with newly diagnosed FLT3-mutated AML and relapsed/refractory IDH2-mutated AML. The next 5 to 10 years will undoubtedly see the emergence of more mutation-specific agents that will effectively create a purely molecular-based treatment algorithm for patients with myeloid malignancies.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110:1092-7.
2. Santamaria CM, Chillon MC, Garcia-Sanz R, et al. Molecular stratification model for prognosis in cytogenetically normal acute myeloid leukemia. Blood. 2009;114:148-52.
3. Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496-506.
4. Stone RM, Mandrekar S, Sanford BL, et al. The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose C consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18-60 with FLT3 mutations (muts): an international prospective randomized (rand) P-controlled double-blind trial (CALGB 10603/RATIFY [Alliance]). Blood. 2015;126:abstr 6.
5. Greenberg P, Cox C, LeBeau MM, et al. International Scoring System for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-88.
6. Greenberg PL, Tuechler H, Schanz J, et al. Revised International Prognostic Scoring System for myelodysplastic syndromes. Blood. 2012;120:2454-65.
7. Kantarjian H, O'Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351-61.
8. Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503-10.
9. Cazzola M, Della Porta MG, Malcovati L. The genetic basis of myelodysplasia and its clinical relevance. Blood. 2013;122:4021-34.
10. Kosmider O, Gelsi-Boyer V, Cheok M, et al. TET2 mutation is an independent favorable prognostic factor in myelodysplastic syndromes (MDSs). Blood. 2009;114:3285-91.
11. Walter MJ, Ding L, Shen D, et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia. 2011;25:1153-8.
12. Bejar R, Stevenson KE, Caughey BA, et al. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2012;30:3376-82.
13. Thol F, Weissinger EM, Krauter J, et al. IDH1 mutations in patients with myelodysplastic syndromes are associated with an unfavorable prognosis. Haematologica. 2010;95:1668-74.
14. Bejar R, Papaemmanuil E, Haferlach T, et al. Somatic mutations in MDS patients are associated with clinical features and predict prognosis independent of the IPSS-R: analysis of combined datasets from the International Working Group for Prognosis in MDS-Molecular Committee. Blood. 2015;126:abstr 907.
15. Heuser M, Koenecke C, Gabdoulline R, et al. Molecular predictors of outcome in patients with MDS and AML following MDS after allogeneic hematopoietic stem cell transplantation. Blood. 2015;126:abstr 912.
16. Chen CY, Lin LI, Tang JL, et al. RUNX1 gene mutation in primary myelodysplastic syndrome--the mutation can be detected early at diagnosis or acquired during disease progression and is associated with poor outcome. Br J Haematol. 2007;139:405-14.
17. Kita-Sasai Y, Horiike S, Misawa S, et al. International Prognostic Scoring System and TP53 mutations are independent prognostic indicators for patients with myelodysplastic syndrome. Br J Haematol. 2001;115:309-12.
18. Horiike S, Kita-Sasai Y, Nakao M, Taniwaki M. Configuration of the TP53 gene as an independent prognostic parameter of myelodysplastic syndrome. Leuk Lymphoma. 2003;44:915-22.
19. Bejar R, Stevenson KE, Caughey B, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol. 2014;32:2691-8.
20. Makishima H, Visconte V, Sakaguchi H, et al. Mutations in the spliceosome machinery, a novel and ubiquitous pathway in leukemogenesis. Blood. 2012;119:3203-10.
21. Malcovati L, Karimi M, Papaemmanuil E, et al. SF3B1 mutation identifies a distinct subset of myelodysplastic syndrome with ring sideroblasts. Blood. 2015;126:233-41.
22. Wu SJ, Kuo YY, Hou HA, et al. The clinical implication of SRSF2 mutation in patients with myelodysplastic syndrome and its stability during disease evolution. Blood. 2012;120:3106-11.
23. Thol F, Kade S, Schlarmann C, et al. Frequency and prognostic impact of mutations in SRSF2, U2AF1, and ZRSR2 in patients with myelodysplastic syndromes. Blood. 2012;119:3578-84.
24. Falini B, Nicoletti I, Martelli MF, Mecucci C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109:874-85.
25. Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079-89.
26. Ivey A, Hills RK, Simpson MA, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;374:422-33.
27. Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074-80.
28. Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2386-92.
29. Feng JH, Guo XP, Chen YY, et al. Prognostic significance of IDH1 mutations in acute myeloid leukemia: a meta-analysis. Am J Blood Res. 2012;2:254-64.
30. Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363:2424-33.
31. Chou WC, Chou SC, Liu CY, et al. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood. 2011;118:3803-10.
32. Ahn JS, Kim HJ, Kim YK, et al. Adverse prognostic effect of homozygous TET2 mutation on the relapse risk of acute myeloid leukemia in patients of normal karyotype. Haematologica. 2015;100:e351-e353.
33. Pabst T, Eyholzer M, Fos J, Mueller BU. Heterogeneity within AML with CEBPA mutations; only CEBPA double mutations, but not single CEBPA mutations are associated with favourable prognosis. Br J Cancer. 2009;100:1343-6.
34. Tang JL, Hou HA, Chen CY, et al. AML1/RUNX1 mutations in 470 adult patients with de novo acute myeloid leukemia: prognostic implication and interaction with other gene alterations. Blood. 2009;114:5352-61.
35. Schnittger S, Dicker F, Kern W, et al. RUNX1 mutations are frequent in de novo AML with noncomplex karyotype and confer an unfavorable prognosis. Blood. 2011;117:2348-57.
36. Stirewalt DL, Kopecky KJ, Meshinchi S, et al. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589-95.
37. Paschka P, Marcucci G, Ruppert AS, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24:3904-11.
38. Metzeler KH, Becker H, Maharry K, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN favorable genetic category. Blood. 2011;118:6920-9.
39. Schnittger S, Eder C, Jeromin S, et al. ASXL1 exon 12 mutations are frequent in AML with intermediate risk karyotype and are independently associated with an adverse outcome. Leukemia. 2013;27:82-91.
40. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209-21.
41. Larrosa-Garcia M, Baer MR. FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther. 2017;16:991-1001.
42. DiNardo C, Stein EM, Altman JK, et al. An oral, selective, first-in-class, potent inhibitor of the IDH2 mutant enzyme, induced durable responses in a phase 1 study of IDH2 mutation-positive advanced hematologic malignancies. Presented at the European Hematology Association 20th Congress; June 11-14, 2015; Vienna, Austria. Abstr P569.
43. de Botton S, Pollyea DA, Stein EM, et al. Clinical safety and activity of AG-120, a first-in-class, potent inhibitor of the IDH1-mutant protein, in a phase 1 study of patients with advanced IDH1-mutant hematologic malignancies. Presented at the European Hematology Association 20th Congress; June 11-14, 2015; Vienna, Austria. Abstr P563.
44. Metzeler KH, Walker A, Geyer S, et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2012;26:1106-7.
45. Sehgal AR, Gimotty PA, Zhao J, et al. DNMT3A mutational status affects the results of dose-escalated induction therapy in acute myelogenous leukemia. Clin Cancer Res. 2015;21:1614-20.
46. Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30:735-41.
47. Kayser S, Dohner K, Krauter J, et al. Impact of allogeneic transplantation from matched related and unrelated donors on clinical outcome in younger adult AML patients with FLT3 internal tandem duplications. Presented at the American Society of Hematology Annual Meeting; December 4-7, 2010; Orlando, Florida. Abstr 909.
48. DeZern AE, Sung A, Kim S, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant. 2011;17:1404-9.
49. Gaidzik VI, Bullinger L, Schlenk RF, et al. RUNX1 mutations in acute myeloid leukemia: results from a comprehensive genetic and clinical analysis from the AML study group. J Clin Oncol. 2011;29:1364-72.
50. Traina F, Visconte V, Elson P, et al. Impact of molecular mutations on treatment response to DNMT inhibitors in myelodysplasia and related neoplasms. Leukemia. 2014;28:78-87.
51. Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124:2705-12.
52. Levine RL, Loriaux M, Huntly BJ, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106:3377-9.
53. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379-90.
54. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472-6.
55. Maxson JE, Gotlib J, Pollyea DA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368:1781-90.
56. Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909-18.
57. Daver N, Strati P, Jabbour E, et al. FLT3 mutations in myelodysplastic syndrome and chronic myelomonocytic leukemia. Am J Hematol. 2013;88:56-9.
58. Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059-74.
59. Boultwood J, Perry J, Pellagatti A, et al. Frequent mutation of the polycomb-associated gene ASXL1 in the myelodysplastic syndromes and in acute myeloid leukemia. Leukemia. 2010;24:1062-5.
60. Haferlach T, Nagata Y, Grossmann V, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241-7.
61. Wang X, Dai H, Wang Q, et al. EZH2 mutations are related to low blast percentage in bone marrow and -7/del(7q) in de novo acute myeloid leukemia. PLoS One. 2013;8:e61341.
62. Ernst T, Chase AJ, Score J, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42:722-6.
63. Barjesteh van Waalwijk van Doorn-Khosrovani S, Spensberger D, de Knegt Y, et al. Somatic heterozygous mutations in ETV6 (TEL) and frequent absence of ETV6 protein in acute myeloid leukemia. Oncogene. 2005;24:4129-37.
64. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118:6153-63.
65. Damm F, Chesnais V, Nagata Y, et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood. 2013;122:3169-77.
66. Wen XM, Hu JB, Yang J, et al. CEBPA methylation and mutation in myelodysplastic syndrome. Med Oncol. 2015;32:192.
67. Hou HA, Liu CY, Kuo YY, et al. Splicing factor mutations predict poor prognosis in patients with de novo acute myeloid leukemia. Oncotarget. 2016;7:9084-101.
68. Makishima H, Yoshida K, Nguyen N, et al. Somatic SETBP1 mutations in myeloid malignancies. Nat Genet. 2013;45:942-6.
69. O'Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
