The Role of Postmastectomy Radiotherapy in Locally Advanced Breast Cancer After Pathological Complete Response to Neoadjuvant Chemotherapy
Experts examine the case of a previously healthy woman, aged 32 years, presented to the oncology clinic with a 6-month history of left-breast tumor, mastalgia, and swollen axillary nodes.
The Case
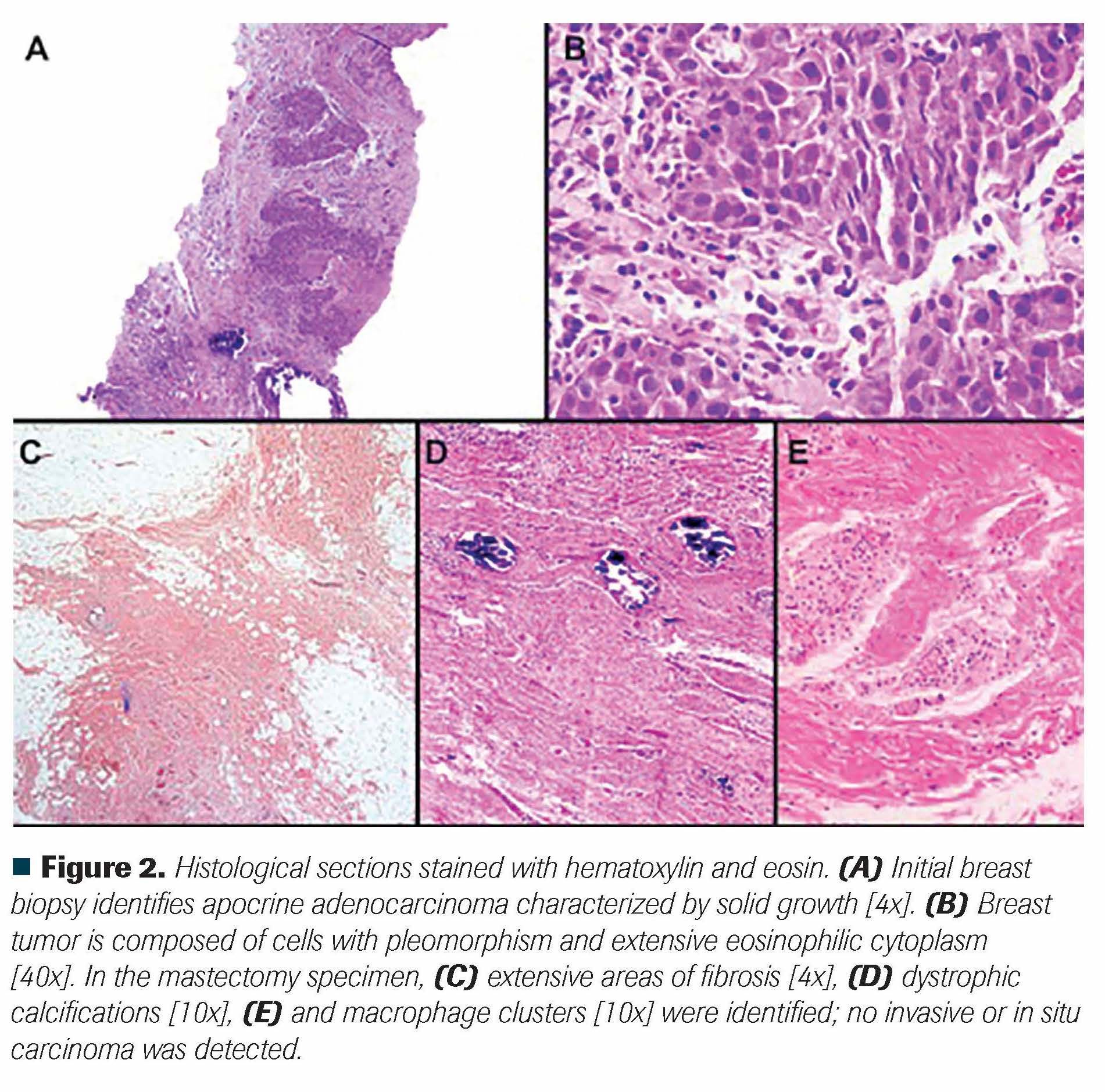
A previously healthy woman, aged 32 years, presented to the oncology clinic with a 6-month history of left-breast tumor, mastalgia, and swollen axillary nodes. Physical examination was relevant for a 6-cm palpable mass in the upper outer quadrant of the left breast and an ipsilateral 2-cm, nonfixed axillary lymph node. Mammography showed a 1-cm mass in the upper outer quadrant, a 5.2-cm mass in the lower outer quadrant, and enlarged pathologic lymph nodes (BI-RADS category 5 disease). Breast ultrasound revealed 3 axillary lymph nodes with cortical thickening and loss of normal morphology (the largest with a 2.6-cm length in the long axis) (Figure 1A-B). The breast's core biopsy revealed a grade 3 apocrine invasive carcinoma with lymphovascular invasion; immunohistochemistry testing showed HER2-negative, hormone receptor-negative disease (estrogen receptor, 0%; progesterone receptor, 0%; HER2-negative, Ki67, 50%) (Figure 2A-B). A fine-needle aspiration biopsy of the axillary lymph nodes showed invasive breast carcinoma as well. Bone scintigraphy and a chest/abdomen CT scan ruled out metastatic disease. Upon initial diagnosis, clinical stage was deemed as cT3N1M0 (American Joint Committee on Cancer 8th edition: anatomic stage IIIA, clinical prognostic stage IIIC).
After a multidisciplinary tumor board discussion, the patient underwent neoadjuvant chemotherapy with weekly paclitaxel, followed by 4 cycles of dosedense doxorubicin plus cyclophosphamide. After completing neoadjuvant treatment, clinical examination was relevant for a residual 1-cm palpable left breast mass and no palpable axillary nodes. Mammography and breast ultrasound showed a 77% partial response in the primary tumors, and axillary nodes with normal morphology and size (Figure 1C-D). Due to multicentric tumor disease, breast-conserving surgery would not confer satisfactory cosmetic results on her, and a modifi ed radical mastectomy with intraoperative sentinel lymph node biopsy (and second-stage breast reconstruction) was planned. However, during surgery, the surgeons failed to identify the mapped lymph node, and level I-III axillary lymph node dissection was performed. The pathology report described complete pathological response: Miller and Payne criteria grade 5 response with the absence of malignant cells within the mastectomy specimen and in 24 lymph nodes (Figure 2C-E). Pathological staging after neoadjuvant treatment concluded ypT0N0M0 disease. Subsequent treatment for this patient was discussed in another tumor board.
Oncology (Williston Park). 2021;35(3):139-143.
DOI: 10.46883/ONC.2021.3503.0139
Figure 1. Imaging findings before (superior) and after (inferior) neoadjuvant chemotherapy. (A) Left mediolateral oblique projection of baseline mammography showing heterogeneous calcifications; 5.2-cm and 1-cm masses located 4 cm and 5 cm from the nipple, respectively (yellow arrows). (B) Left axilla basal ultrasound revealing enlarged pathologic lymph nodes (red arrows). (C) Left mediolateral oblique projection of follow-up mammography showing partial response in breast masses. (D) Left axilla follow-up ultrasound showing a lymph node with preserved morphology and size (red arrow).

Figure 2. Histological sections stained with hematoxylin and eosin. (A) Initial breast biopsy identifies apocrine adenocarcinoma characterized by solid growth [4x]. (B) Breast tumor is composed of cells with pleomorphism and extensive eosinophilic cytoplasm [40x]. In the mastectomy specimen, (C) extensive areas of fibrosis [4x], (D) dystrophic calcifications [10x], (E) and macrophage clusters [10x] were identified; no invasive or in situ carcinoma was detected.
Considering the complete pathological response achieved, what is the best treatment option for this patient after neoadjuvant chemotherapy and surgery?
A. Surveillance only
B. Adjuvant chemotherapy with capecitabine
C. Adjuvant radiotherapy to the chest wall and regional lymph nodes
D. Adjuvant radiotherapy to the chest wall only
Discussion
Worldwide, breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death in women1; regional and distant recurrences occur even when appropriate breast and axillary surgery is performed, thus compromising survival.2 In patients with a high risk of recurrence after mastectomy (involvement of axillary lymph nodes, tumor size >5 cm, invasion to the skin, or pectoral fascia), radiation therapy is indicated.3 Following upfront surgery and adjuvant chemotherapy, postmastectomy radiation therapy (PMRT; radiation to the chest wall and regional lymph nodes) decreases the rate of locoregional recurrence (LRR) and increases long-term disease-free survival (DFS), cancer-specific survival (CSS), and overall survival (OS).4 These benefits have been reported in multiple trials and meta-analyses.5-9
The benefits mentioned above have not been consistently demonstrated when neoadjuvant chemotherapy (NACT) is used.10 The primary goal of NACT is to induce tumor response before surgery and enable breast conservation.11 NACT results in long-term distant metastasis-free survival (DMFS) and OS similar to those achieved with primary surgery followed by adjuvant systemic therapy.12,13 Pathologic complete response (pCR) after NACT is associated with improved OS in breast cancer patients14; this association persists even when pCR is limited to either the breast (ypT0) or nodal (ypN0) compartments.15,16 When analyzing by breast cancer subtypes, patients with triple-negative and HER2-positive, hormone receptor-negative disease consistently achieve significant OS benefit, while this does not hold true for hormone receptor-positive tumors (whether they are HER2-positive or negative).14,15 To date, it is unclear how the response to NACT should be incorporated into decisions regarding locoregional adjuvant therapy.17 Data from ongoing NACT trials are still pending (NCT01872975; NCT01901094).18,19
None of the prospective phase 3 trials yet conducted have investigated the impact of omitting PMRT in patients who received NACT and develop certain tumor response (ypN0 or pCR). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 NACT trials were performed when PMRT benefit was unknown and thus prohibited; these studies are a unique way to study the pattern of recurrence following NACT.20,21 A combined analysis of such trials reported that the axillary nodal response to NACT (ypN0) yielded low LRR rates without the use of PMRT, even in initially involved cases (cN+), thus supporting the hypothesis that a NACT response selects a lower-risk group that does not receive benefit from the addition of PMRT.22 Therefore, ypN0 (vs ypN+) is the usual way in which NACT studies report their findings.
Retrospective studies show discordant results of PMRT in the NACT setting. Data from MD Anderson Cancer Center showed that clinical stage II-III patients had a lower rate of LRR when PMRT was used (10-year LRR, 11% vs 22%; P = .0001), despite the fact that more advanced disease was present in the PMRT group; patients with clinical stage III-IV disease with complete response still had benefited from PMRT.23
An analysis of 3 prospective German trials showed that PMRT was associated with a lower risk of LRR, especially in patients with cT3/T4 and cN+ tumors; patients who converted from cN+ to ypN0 had significantly lower LRR risk with PMRT (HR, 0.19; 95% CI, 0.04-0.97; P = .05). No benefit in DFS was noted in patients who were clinically node positive at diagnosis and received RT.24 A French study investigated the outcomes of 134 women with clinical stage II-III disease treated with NACT and ypN0 disease evidenced in axillary dissection specimens. PMRT was not associated with significant differences in LRR-free survival or OS.25 A multicenter study of 417 similar patients in Korea also failed to show benefit from PMRT in LRR, DFS, or OS.26
National database studies also have divergent conclusions. A study based on data from the Japanese Breast Cancer Registry examined 3226 patients with cT1-4 cN0-2 tumors with NACT and mastectomy and the association between PMRT and LRR and OS based on ypN status. PMRT was not associated with significant differences in 5-year LRR-free survival or 5-year OS for ypN0 and ypN1 patients; only the ypN2-3 cohort showed benefit in the aforementioned outcomes.27 A similar analysis of data from the United States’ National Clinical Data Base (NCDB) examined the outcomes of 15,315 cT1-3 cN1 patients with NACT and either mastectomy or breast-conserving surgery. For the mastectomy patients (N = 10,283), PMRT was independently associated with improved OS for both the ypN0 (HR, 0.729; 95% CI, 0.566-0.939; P = .015) and ypN1 (HR, 0.772; 95% CI, 0.689-0.866; P < .001) groups.28
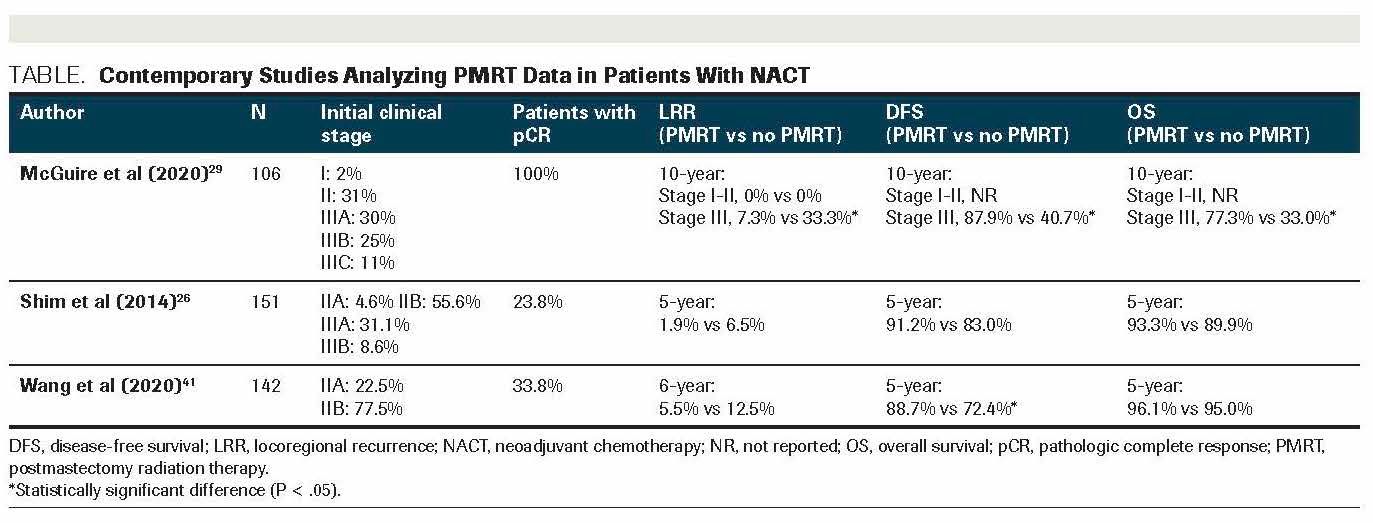
The benefit of PMRT when achieving pCR is more difficult to ascertain, as most of the studies are underpowered to show significant differences in this subgroup with relatively good prognoses. The only retrospective study that analyzed this population comes from MD Anderson Cancer Center, in which the outcome of 226 stage I-III patients and a pCR to NACT was examined: While PMRT did not affect the 10-year LRR of patients with stage I-II disease, patients with stage III disease saw a significant decrease in the 10-year LRR (7.3% vs 33.3%; P = .04), and significant increases in DMFS (87.9% vs 40.7%; P = .0006), CSS (87% vs 40%; P = .0014), and OS (77.3% vs 33.3%; P = .0016) were achieved by PMRT.29 In an analysis of patients with pCR (N = 275) in the Taiwan Cancer Registry, PMRT was associated with improved LRR-free survival but not with DMFS or OS.30 Further data from these and other relevant studies are listed in the Table.
TABLE. Contemporary Studies Analyzing PMRT Data in Patients With NACT
Two ongoing phase 3 studies, NSABP B-51 and Alliance A011202, are investigating the role of adjuvant radiotherapy in ypN0 and ypN+ patients, respectively.18,19 Results from these trials will help clarify if adjuvant radiotherapy can be safely omitted in these subsets of patients after NACT, and they are highly anticipated. However, while results from the aforementioned trials are not yet available, the 2021 National Comprehensive Cancer Network (NCCN) clinical guidelines state that in patients with NACT, adjuvant radiotherapy should be based on the maximal disease stage irrespective of tumor response to NACT.31 Therefore, based on the current NCCN guidelines, answer (a) is incorrect: Patients with locally advanced disease at diagnosis should receive adjuvant radiotherapy even with an excellent response to NACT. Surveillance omits potential benefits in LRR, DMFS, and OS.31 Regarding adjuvant capecitabine, available data support its use only when residual invasive disease is present after NACT, as it offers an improvement in OS. Such benefit is independent of the one provided by radiotherapy.32 Currently, no data support adjuvant capecitabine in patients who achieve a pCR. Therefore, answer (b) is incorrect.
Most recently, tumor biology has been evaluated as an essential factor to consider when thinking about the omission of PMRT. Hormone receptor-positive tumors are associated with lesser LRR and better OS; the relationship between HER2 positivity and different cancer outcomes has not consistently been demonstrated.23,24,30 In a post hoc analysis of the ACOSOG Z1071 NACT trial, the omission of PMRT was associated with a higher rate of LRR in cT0-T4cN1-2 patients. Patients with triple-negative tumors had higher LRR rates than hormone receptor–positive patients (HR, 3.46; 95% CI, 1.68-7.13). Among this subgroup, patients with PMRT had numerically greater LRRfree rates compared with no PMRT (91.0% vs 77.9%); the difference was not statistically significant likely due to the small sample.33 In another analysis from the NCDB, patients with cN1 disease and ypN0 tumors did not have an OS benefit with PMRT; however, among the hormone receptor–negative subgroup, patients had an OS improvement (HR, 0.65; P < .01).34
Even in triple-negative disease, several other prognostic factors must be taken into account. In a study of 390 patients in China with triple-negative, stage I-III disease without PMRT, predictive factors for any LRR were studied. On multivariate analysis, 4 risk factors were associated with increased risk of recurrence: aged <50 years, lymphovascular invasion, high-grade tumor, and 3 or more involved lymph nodes. The 5-year LRR rate for patients who had 0 or 1, 2, and 3 or 4 risk factors were 4.2%, 25.2%, and 81.0%, respectively (P <.0001).35 The patient from the present case had 3 of those risk factors. It is worth mentioning that the adverse outcome of triple-negative disease can potentially be mitigated by PMRT. In a prospective controlled trial, patients with triple-negative, stage I-II disease who had modified radical mastectomy were randomized to chemotherapy or chemotherapy followed by PMRT. The addition of PMRT significantly increased the 5-year DFS (88.3% vs 74.6%; P = .02) and OS (90.4% vs 78.7%; P = .03).36 Notably, the 2 above-mentioned trials were not specific to patients receiving NACT.
The value of adding regional node irradiation (RNI) to chest wall irradiation with up-front surgery and adjuvant chemotherapy has been addressed by several randomized trials, demonstrating consistent benefit in LRR, DFS, and CSS. No improvement in OS has been shown on a single-study basis.37,38 A meta-analysis of these and other studies (N = 10,620 patients) showed that adding radiation therapy to supraclavicular and internal mammary lymph nodes (vs radiation to the chest wall only) improves LRR, DMFS, and OS.39
Information about RNI in addition to chest wall radiotherapy when using NACT is scarce. Most of the previously described studies used radiotherapy to the chest wall and ”regional nodes at risk” without properly describing which regions were irradiated.23-27,29,30,33,34 A retrospective study from MD Anderson Cancer Center explored the benefit of RNI in addition to radiation to the chest wall/breast in node-positive breast cancer treated with NACT. RNI was defined as radiation therapy to at least 1 of the regional draining lymphatic basins at the radiation oncologist´s discretion. On multivariate analysis, RNI significantly reduced the risk of LRR (HR, 0.497; 95% CI, 0.279-0.884; P = .02); no benefit to CSS or OS was seen.40
NCCN guidelines state that in patients with N1 disease, radiation therapy to chest wall, infraclavicular and supraclavicular regions, internal mammary chain plus any part of the axillary bed at risk should be strongly considered. 31 In the last Saint Gallen consensus, 85% of participants agreed that radiation to the chest wall plus regional nodes would be the optimal choice for patients with N1 disease when adverse features are present (ie, triple-negative disease).41 Therefore answer (d) is incorrect: Radiation fields in locally advanced, high-risk disease should include regional lymph nodes in addition to the chest wall. Thus, (c) is the correct answer.
Outcome of This Case
The patient received PMRT (50 Gy in 25 fractions) to the chest wall and regional lymph nodes (axillary levels I-III, supraclavicular, and internal mammary) because of the high-risk factors of her disease. The patient has been under follow-up during the past 16 months without evidence of locoregional recurrence or distant metastases; her breast reconstruction surgery is being planned.
Note: Due to the absence of randomized controlled trials that determine the effect of PMRT in patients with NACT who achieve pCR, the authors highlight the necessity of multidisciplinary team evaluation for each patient. This case represents the authors’ opinion of how this particular patient should be treated off-trial. The ongoing randomized studies will elucidate whether this is ultimately the correct approach.
CORRECT ANSWER: C. Adjuvant radiotherapy to the chest wall and regional lymph nodes.
FINANCIAL DISCLOSURE: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Flores-Balcázar is an associate professor, national researcher and head of the Radiotherapy and Medical Physics Service at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.
Castro-Alonso is a clinical resercher at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.
Hernández-Barragán is an Advanced Radiotherapy Fellow at Institut Català d’Oncologia, Barcelona, Spain.
Delgado-de la Mora is a pathology fellow at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.
Daidone is head of the Radiotherapy Service at Centro di Medicina Nucleare San Gaetano / Villa Santa Teresa, Sicily, Italy.
Trejo-Durán is an associate professor at Instituto Nacional de Cancerología, Mexico City.
References:
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Published online February 4, 2021. CA Cancer J Clin. doi:10.3322/caac.21660
2. Buchanan CL, Dorn PL, Fey J, et al. Locoregional recurrence after mastectomy: incidence and outcomes. J Am Coll Surg. 2006;203(4):469-474. doi:10.1016/j.jamcollsurg.2006.06.015
3. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135. doi:10.1016/S0140-6736(14)60488-8. Published correction appears in Lancet. 2014;384(9957):1848.
4. Danish Breast Cancer Cooperative Group, Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24(15):2268-2275. doi:10.1200/JCO.2005.02.8738
5. Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(2):116-126. doi: 10.1093/jnci/djh297
6. Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b trial. N Engl J Med. 1997;337(14):949-955. doi:10.1056/NEJM199710023371401
7. Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337(14):956-962. doi:10.1056/NEJM199710023371402
8. Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641-1648. doi:10.1016/S0140-6736(98)09201-0
9. Clarke M, Collins R, Darby S, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087-2106. doi:10.1016/S0140-6736(05)67887-7
10. Teshome M, Hunt KK. Neoadjuvant therapy in the treatment of breast cancer. Surg Oncol Clin N Am. 2014;23(3):505-523. doi:10.1016/j.soc.2014.03.006
11. Guarneri V, Dieci MV, Barbieri E, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013;24(12):2990-2994. doi:10.1093/annonc/mdt364
12. Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol. 2012;23 (Suppl 10),x231-x236. doi:10.1093/annonc/mds324
13. Cance WG, Carey LA, Calvo BF, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg. 2002;236(3):295-302; discussion 302-303. doi:10.1097/01.SLA.0000027526.67560.64
14. Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26(12):2838-2848. doi:10.1158/1078-0432.CCR-19-3492
15. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi:10.1016/S0140-6736(13)62422-8. Published correction appears in Lancet. 2019;393(10175):986.
16. Fayanju OM, Ren Y, Thomas SM, et al. The clinical significance of breast-only and node-only pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT): a review of 20,000 breast cancer patients in the National Cancer Data Base (NCDB). Ann Surg. 2018;268(4):591-601. doi:10.1097/SLA.0000000000002953
17. Hunter Chapman C, Jagsi R. Postmastectomy radiotherapy after neoadjuvant chemotherapy: a review of the evidence. Oncology(Williston Park). 2015;29(9):657-666.
18. Standard or comprehensive radiation therapy in treating patients with early-stage breast cancer previously treated with chemotherapy and surgery. ClinicalTrials.gov. Updated August 26, 2019. Accessed October 6, 2020. https://clinicaltrials.gov/ct2/show/NCT01872975
19. Comparison of axillary lymph node dissection with axillary radiation for patients with node-positive breast cancer related with chemotherapy. ClinicalTrials.gov. Updated February 8, 2021. Accessed February 11, 2021 https://clinicaltrials.gov/ct2/show/NCT01901094
20. Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;(30):96-102. doi:10.1093/oxfordjournals.jncimonographs.a003469
21. Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24(13):2019-2027. doi:10.1200/JCO.2005.04.1665
22. Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30(32):3960-3966. doi:10.1200/JCO.2011.40.8369
23. Huang EH, Tucker SL, Strom EA, et al. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol. 2004;22(23):4691-4699. doi:10.1200/JCO.2004.11.129. Published correction appears in J Clin Oncol. 2005;23(1):248.
24. Krug D, Lederer B, Seither F, et al. Post-mastectomy radiotherapy after neoadjuvant chemotherapy in breast cancer: a pooled retrospective analysis of three prospective randomized trials. Ann Surg Oncol. 2019;26(12):3892-3901. doi:10.1245/s10434-019-07635-x
25. Le Scodan R, Selz J, Stevens D, et al. Radiotherapy for stage II and stage III breast cancer patients with negative lymph nodes after preoperative chemotherapy and mastectomy. Int J Radiat Oncol Biol Phys. 2012;82(1):e1-e7. doi:10.1016/j.ijrobp.2010.12.054
26. Shim SJ, Park W, Huh SJ, et al. The role of postmastectomy radiation therapy after neoadjuvant chemotherapy in clinical stage II-III breast cancer patients with pN0: a multicenter, retrospective study (KROG 12-05). Int J Radiat Oncol Biol Phys. 2014;88(1):65-72. doi:10.1016/j.ijrobp.2013.09.021
27. Miyashita M, Niikura N, Kumamaru H, et al. Role of postmastectomy radiotherapy after neoadjuvant chemotherapy in breast cancer patients: a study from the Japanese Breast Cancer Registry. Ann Surg Oncol. 2019;26(8):2475-2485. doi:10.1245/s10434-019-07453-1
28. Rusthoven CG, Rabinovitch RA, Jones BL, et al. The impact of postmastectomy and regional nodal radiation after neoadjuvant chemotherapy for clinically lymph node-positive breast cancer: a National Cancer Database (NCDB) analysis. Ann Oncol. 2016;27(5):818-827. doi:10.1093/annonc/mdw046
29. McGuire SE, Gonzalez-Angulo AM, Huang EH, et al. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68(4):1004-1009. doi:10.1016/j.ijrobp.2007.01.023
30. Zhang J, Lu CY, Chen CH, Chen HM, Wu SY. Effect of pathologic stages on postmastectomy radiation therapy in breast cancer receiving neoadjuvant chemotherapy and total mastectomy: a Cancer Database Analysis. Breast. 2020;54:70-78. doi:10.1016/j.breast.2020.08.017
31. NCCN. Clinical Practice Guidelines in Oncology. Breast cancer, version 1.2021. Accessed January 15, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
32. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi:10.1056/NEJMoa1612645
33. Haffty BG, McCall LM, Ballman KV, Buchholz TA, Hunt KK, Boughey JC. Impact of radiation on locoregional control in women with node-positive breast cancer treated with neoadjuvant chemotherapy and axillary lymph node dissection: results from ACOSOG Z1071 clinical trial. Int J Radiat Oncol Biol Phys. 2019;105(1):174-182. doi:10.1016/j.ijrobp.2019.04.038
34. Kantor O, Pesce C, Singh P, et al. Post-mastectomy radiation therapy and overall survival after neoadjuvant chemotherapy. J Surg Oncol. 2017;115(6):668-676. doi:10.1002/jso.24551
35. Chen X, Yu X, Chen J, et al. Analysis in early stage triple-negative breast cancer treated with mastectomy without adjuvant radiotherapy: patterns of failure and prognostic factors.Cancer. 2013;119(13):2366-2374. doi:10.1002/cncr.28085
36. Wang J, Shi M, Ling R, et al. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: a prospective randomized controlled multi-center trial. Radiother Oncol. 2011;100(2):200-204. doi:10.1016/j.radonc.2011.07.007
37. Whelan TJ, Olivotto IA, Levine MN. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(19):1878-1879. doi:10.1056/NEJMc1510505
38. Poortmans PM, Weltens C, Fortpied C, et al; European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol. 2020;21(12):1602-1610. doi:10.1016/S1470-2045(20)30472-1. Published correction appears in Lancet Oncol. 2021;22(1):e5.
39. Haussmann J, Budach W, Tamaskovics B, et al. Which target volume should be considered when irradiating the regional nodes in breast cancer? results of a network-meta-analysis. Radiat Oncol. 2019;14(1):102. doi:10.1186/s13014-019-1280-6
40. Stecklein SR, Park M, Liu DD, et al. Long-term impact of regional nodal irradiation in patients with node-positive breast cancer treated with neoadjuvant systemic therapy. Int J Radiat Oncol Biol Phys. 2018;102(3):568-577. doi:10.1016/j.ijrobp.2018.06.016
41. Balic M, Thomssen C, Würstlein R, Gnant M, Harbeck N. St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care (Basel). 2019;14(2):103-110. doi:10.1159/000499931
