Factors Associated With Treatment Refusal and Impact of Treatment Refusal on Survival of Patients With Small Cell Lung Cancer
Original research published in the journal ONCOLOGY® explored the impacts of treatment refusal in patients with small cell lung cancer.
ABSTRACT
BACKGROUND: With fewer than 7% of patients with small cell lung cancer (SCLC) surviving 5 years after diagnosis, the receipt of recommended treatment is of utmost importance for patient survival. Nevertheless, treatment refusal by patients with SCLC has not been studied well. Our study examined factors associated with treatment refusal and the effect of refusal on patient survival.
METHODS: From the National Cancer Database, we analyzed data of 107,988 patients with SCLC diagnosed between 2003 and 2012. Treatment refusals were analyzed separately for chemoradiotherapy among patients with limited-stage disease (LS-SCLC) and chemotherapy among those with extensive-stage disease (ES-SCLC). We used logistic regression to investigate factors associated with treatment refusal. We estimated survival probability using the Kaplan–Meier method and compared survival of those who received and refused treatment using Cox proportional hazards regression analysis.
RESULTS: The refusal rates of chemoradiotherapy among patients with LSSCLC and chemotherapy among those with ES-SCLC were 1.34% and 4.70%, respectively. From 2003 to 2012, trends show an increase of refusals, especially among the patients with ES-SCLC who were recommended chemotherapy. Multivariable analyses showed that in both SCLC groups, older age at diagnosis (>70 years), female gender, uninsured status, and presence of comorbidities were associated with treatment refusals. Patients with LS-SCLC who refused chemoradiotherapy had a higher risk of mortality than those who received treatment (HR, 4.96; 95% CI, 4.45-5.53); the median survival of those who refused treatment was 3 months vs 18 months for those who received it (P <.001). Similarly, patients with ES-SCLC who refused chemotherapy had a higher risk of mortality than those who received treatment (HR, 3.69; 95% CI, 3.48-3.92); the median survival was 1 month vs 7 months, respectively (P < .001).
CONCLUSIONS: Treatment refusal among patients with SCLC was associated with worse survival. Strategies to increase patient acceptance of the recommended treatment need to be studied further.
KEYWORDS: Treatment, refusal, small cell lung cancer, survival
Oncology (Williston Park). 2021;35(3):111-118.
DOI: 10.46883/ONC.2021.3503.0111
Deviany is a senior researcher at the Center for Family Welfare & lecturer at Faculty of Public Health, University Indonesia, Depok Campus, West Java, Indonesia.

Ganti is a staff physician at VA Nebraska Western Iowa Health Care System as well as a professor of medicine in the Division of Oncology-Hematology and professor (Courtesy) of Biochemistry and Molecular Biology at the University of Nebraska Medical Center.

Islam is an associate professor of epidemiology and senior program director of, MPH Program, Institute of Public and Preventive Health, Department of Population Health Sciences, Medical College of Georgia, Augusta University.

Introduction
Of the estimated 234,030 new lung cancer cases diagnosed in the United States in 2018, more than 30,000 cases were identified as small cell lung cancer (SCLC).1 Analyses of Surveillance, Epidemiology, and End Results Program (SEER) data indicated that the 5-year survival rate of SCLC was less than 7%.1 In addition, in the last 2 decades, the SCLC survival rate has shown little improvement.2
Therapeutic options for SCLC have not significantly changed in the last 30 years.3,4 Concurrent chemoradiotherapy is typically recommended for patients diagnosed with limited-stage SCLC (LS-SCLC), whereas those diagnosed with extensive- stage SCLC (ES-SCLC) are treated predominantly with chemotherapy.3,5,6 Lack of patient acceptance of recommended treatment has been implicated by previous studies as a key factor in SCLC survival.3,4,7 Nonetheless, there is a lack of research concerning treatment refusal among patients with SCLC.4,8
Our study addressed this gap by examining factors associated with treatment refusal by patients with SCLC in a US national facility-based cancer registry. We also analyzed the trend of treatment refusal over time as well as the effect of treatment refusal on patient survival.
Materials and Methods
Study Population, Design, and Data Source
The National Cancer Database (NCDB) provides national cancer surveillance data through a joint program of the American College of Surgeons’ Commission on Cancer (CoC) and the American Cancer Society (ACS).9 This database captures approximately 70% of newly diagnosed cancer cases.9,10
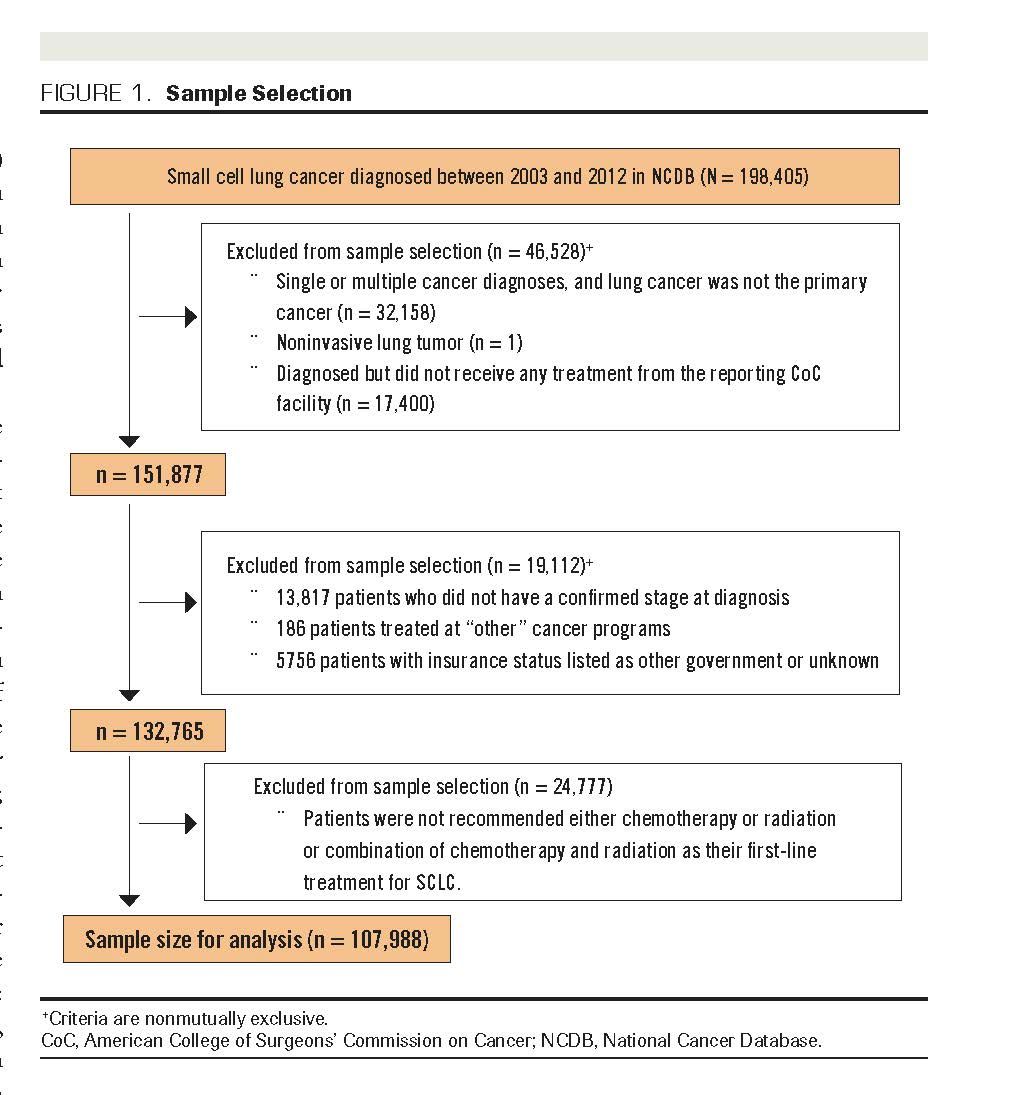
We used cross-sectional design in the analysis of factors associated with treatment refusal and retrospective cohort design in the analysis of survival data. The samples for analysis were drawn from the 198,405 SCLC cases diagnosed between 2003 and 2012. Figure 1 shows the sample selection process. Criteria for data inclusion were: (1) confirmed diagnosis of primary invasive SCLC according to the International Classification of Disease for Oncology, Third Edition codes 8041-8045; (2) diagnosis established at a CoC-accredited facility, and patient received all or part of their treatment from 1 or more CoC facilities; (3) diagnosis confirmed with cancer stage, and patient was treated at 1 or more of the following types of CoC facilities: community, comprehensive community, and academic cancer programs; (4) known insurance status; and (5) recommendation for first line of treatment was either chemotherapy or radiation or combination of chemoradiotherapy. We utilized the data of 107,988 eligible patients in our analyses.
FIGURE 1. Sample Selection
Exposures and Outcomes
We included sociodemographic and clinical characteristics as predictor variables. Information on cancer stage was taken from the NCDB analytic stage, which uses standards publicized by the American Joint Committee on Cancer (7th edition).9 In the analysis, we followed the recommendation of the International Association for the Study of Lung Cancer by grouping stages I to III as LS-SCLC and stage IV as ESSCLC.11 Comorbidities were calculated using the Charlson Comorbidity Index.12 We used the NCDB definition of median income quartile according to the proportion of income range in the patient’s area of residence for income categories. Residence category was based on those established by the United States Department of Agriculture Economic Research Service.9
Our outcome variables were refusal of recommended treatment and 5-year overall mortality. Refusal of recommended treatment was defined as standard therapies recommended by a physician(s) and refused by the patient. We analyzed data of those patients with LS-SCLC who were recommended chemoradiotherapy and of those with ES-SCLC who were recommended chemotherapy. Our analysis focused particularly on treatment refusal by patients who were recommended the above therapies, through the comparison of their median survival to those who accepted recommended treatment. In addition, we examined the effect of treatment refusal on patient survival in the multivariable model.
Statistical Analysis
The χ2 test was used to examine differences in demographic and clinical characteristics of patients with LS-SCLC and ES-SCLC who refused and received the recommended treatments. We used multivariable logistic regression to examine factors associated with refusal of chemoradiotherapy among patients with LS-SCLC and refusal of chemotherapy among patients with ES-SCLC. Backward selection was used to fit the multivariable logistic regression model. Changes in the proportion of treatment refusal over time for cases diagnosed between 2003 and 2012 are shown graphically in Figure 2. We used the Kaplan-Meier method to estimate survival probabilities and log-rank test to assess survival differences. We performed multivariable Cox proportional hazards (PH) regression to examine the effect of treatment refusal on the risk of mortality, adjusted for other factors. The PH assumption was tested using log–log plots; all predictors met the proportionality assumption. Analyses were performed using SAS software version 9.4 (SAS Institute; Cary, NC).
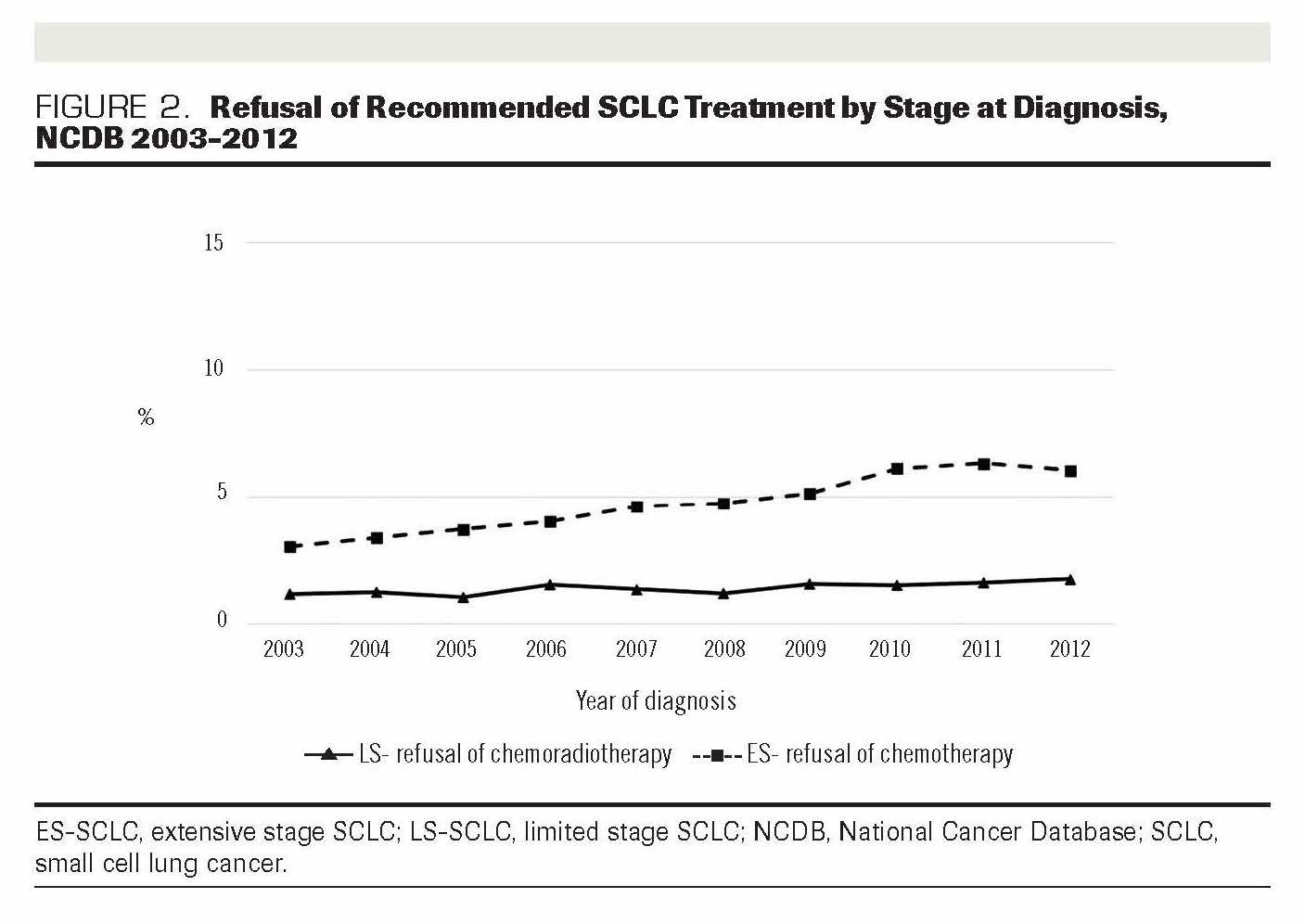
FIGURE 2. Refusal of Recommended SCLC Treatment by Stage at Diagnosis, NCDB 2003-2012
Confidentiality and Ethics
We used data from a deidentified NCDB file. The ACS and CoC have not verified, and neither is responsible for, the analytic and statistical methods utilized in this study. The statements made in the discussion and the conclusions drawn from these data are solely the authors’ responsibility. This study has been classified as exempt by the University of Nebraska Medical Center’s Institutional Review Board due to the use of deidentified data.
Results
Demographic and Clinical Characteristics
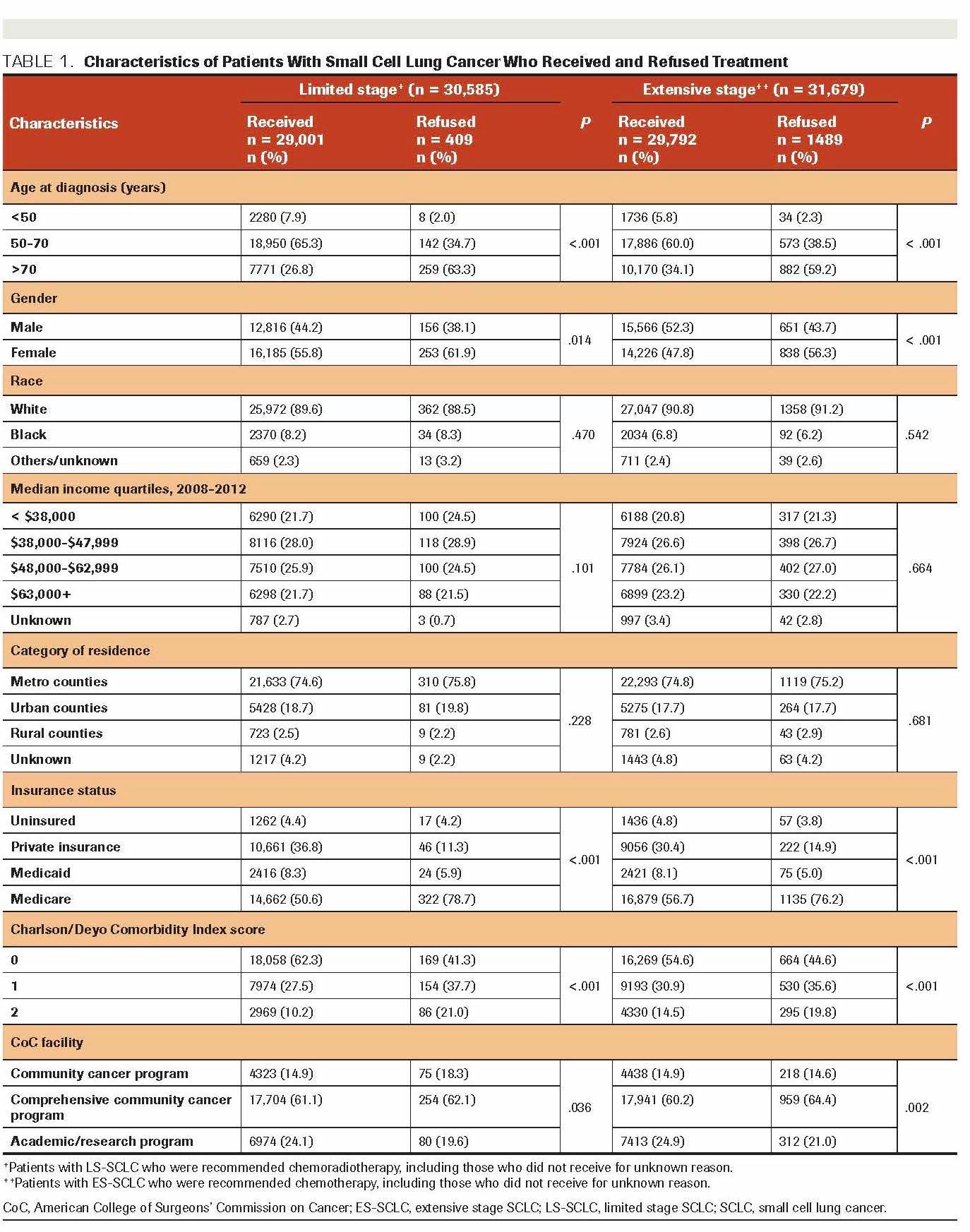
Of the 107,988 SCLC cases included in the analysis, 40,432 patients were diagnosed with LS-SCLC; and 67,556 with ES-SCLC. Comparison of characteristics of patients who received and refused treatment for LS-SCLC and ES-SCLC are presented in Table 1. Separate analyses among those with LS-SCLC and ES-SCLC showed that higher proportions of patients with older age at onset (>70 years), women, and patients treated in a comprehensive community cancer program refused the recommended treatment modalities (P < .001). Interestingly, those without comorbidities had a higher proportion of refusal than those with comorbidities. For the first-line treatment (data not shown), chemoradiotherapy was recommended to 75% of the patients with LS-SCLC (n = 40,432). Among patients diagnosed with ES-SCLC (n = 67,556), chemotherapy and chemoradiotherapy were recommended to 47% and 45%, respectively.
TABLE 1. Characteristics of Patients With Small Cell Lung Cancer Who Received and Refused Treatment
Trend of Treatment Refusal
In total, 1898 patients declined the recommended chemoradiotherapy for LS-SCLC and the recommended chemotherapy for ES-SCLC. Figure 2 shows the increase in the proportion of refusal of each therapy over 10 years. Overall, refusal of the recommended chemoradiotherapy among patients diagnosed with LS-SCLC was 1.34% and refusal of the recommended chemotherapy among patients with ES SCLC was 4.70%. Analysis of 10 years’ worth of data shows that the increase in refusal among those with ES-SCLC was higher (50%) than it was among those with LS-SCLC (34%).
Factors Associated With Treatment Refusal
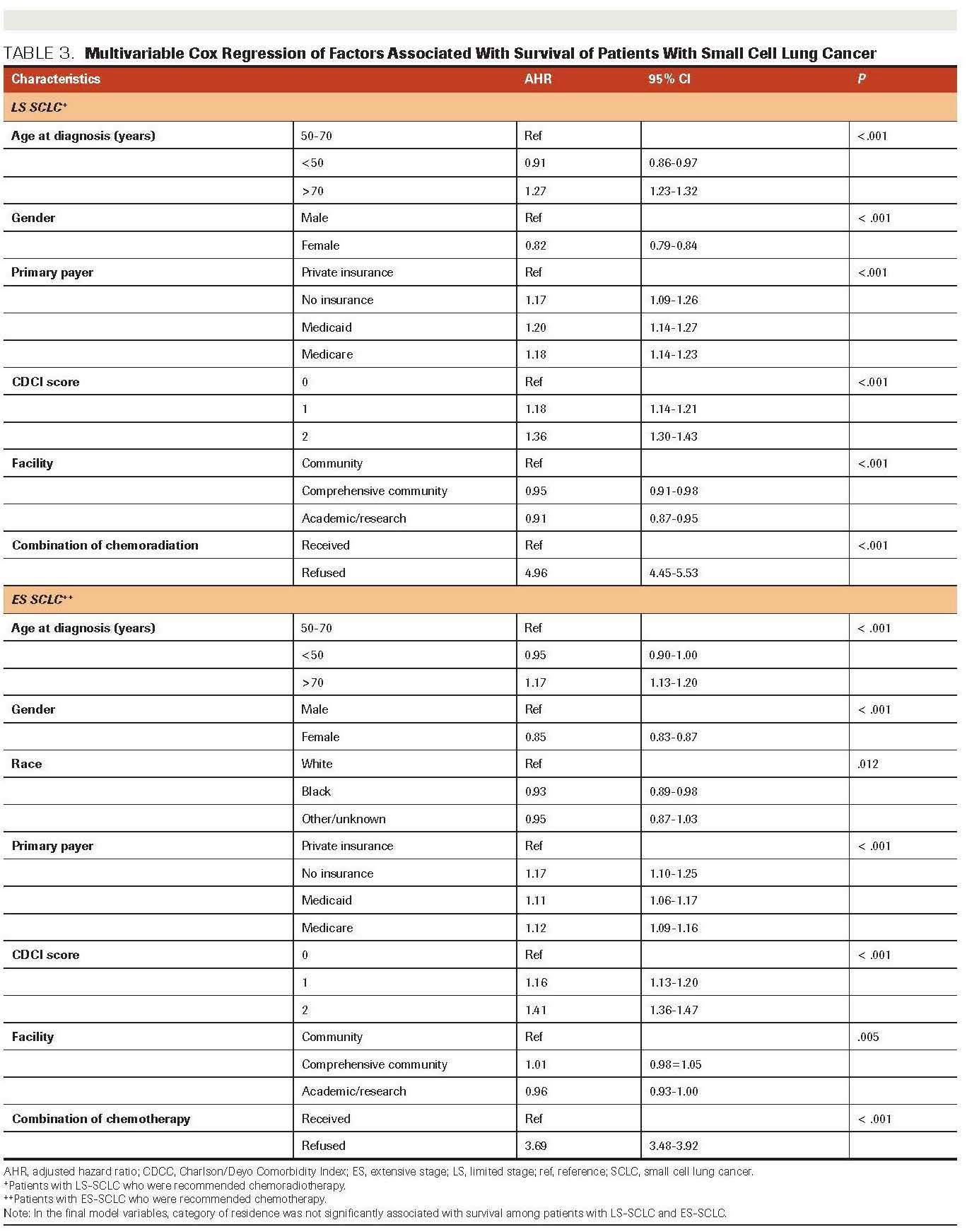
After adjusting for demographic and clinical characteristics, we found that age at diagnosis, gender, insurance status, and comorbidity score were associated with the refusal of chemoradiotherapy among patients with LS-SCLC (Table 2). Patients diagnosed when aged >70 years were more likely to refuse treatment than those aged 50 to 70 years; the adjusted odds ratio (AOR) for refusal of chemoradiotherapy was 3.50 (95% CI, 2.77-4.44). Women were more likely to refuse recommended chemoradiotherapy than men (AOR, 1.29; 95% CI, 1.05-1.58). Compared with those with private insurance, uninsured patients were more likely to refuse treatment (AOR, 3.61; 95% CI, 2.05-6.35). In addition, we found that patients with comorbid conditions were more likely to refuse recommended chemoradiotherapy than those without comorbidities (AOR, 2.54; 95% CI, 1.94-3.31).
The multivariable logistic regression analysis showed that factors similar to those observed in the analysis of LSSCLC were associated with refusal of chemotherapy among patients with ES-SCLC (Table 3). Patients diagnosed when aged >70 years were more likely to refuse chemotherapy than those aged 50 to 70 years (AOR, 2.25; 95% CI, 1.99-2.55). Women were more likelyto refuse recommended treatment than men (AOR, 1.42; 95% CI, 1.27-1.58). Uninsured patients were more likely to refuse chemotherapy than those with private insurance (AOR, 1.76; 95% CI, 1.29-2.40). Patients with ES-SCLC with comorbidities were more likely to refuse chemotherapy than patients without comorbidities (AOR; 1.43; 95% CI, 1.23-1.65). As previous studies had suggested,13,14 interactions of race with variables including gender, insurance status, and comorbidity were examined separately for both LS-SCLC and ESSCLC, but no significant association was found. We used backward selection to fit the multivariable logistic regression model, with cutoff 0.2 to be included in the model and 0.05 to stay in the model. Also, we tested the interaction among variables in the model such as race, gender, insurance status, and comorbidity and found no significant association.
TABLE 2. Multivariable Logistic Regression of Factors Associated With Refusal of Small Cell Lung Cancer Treatment
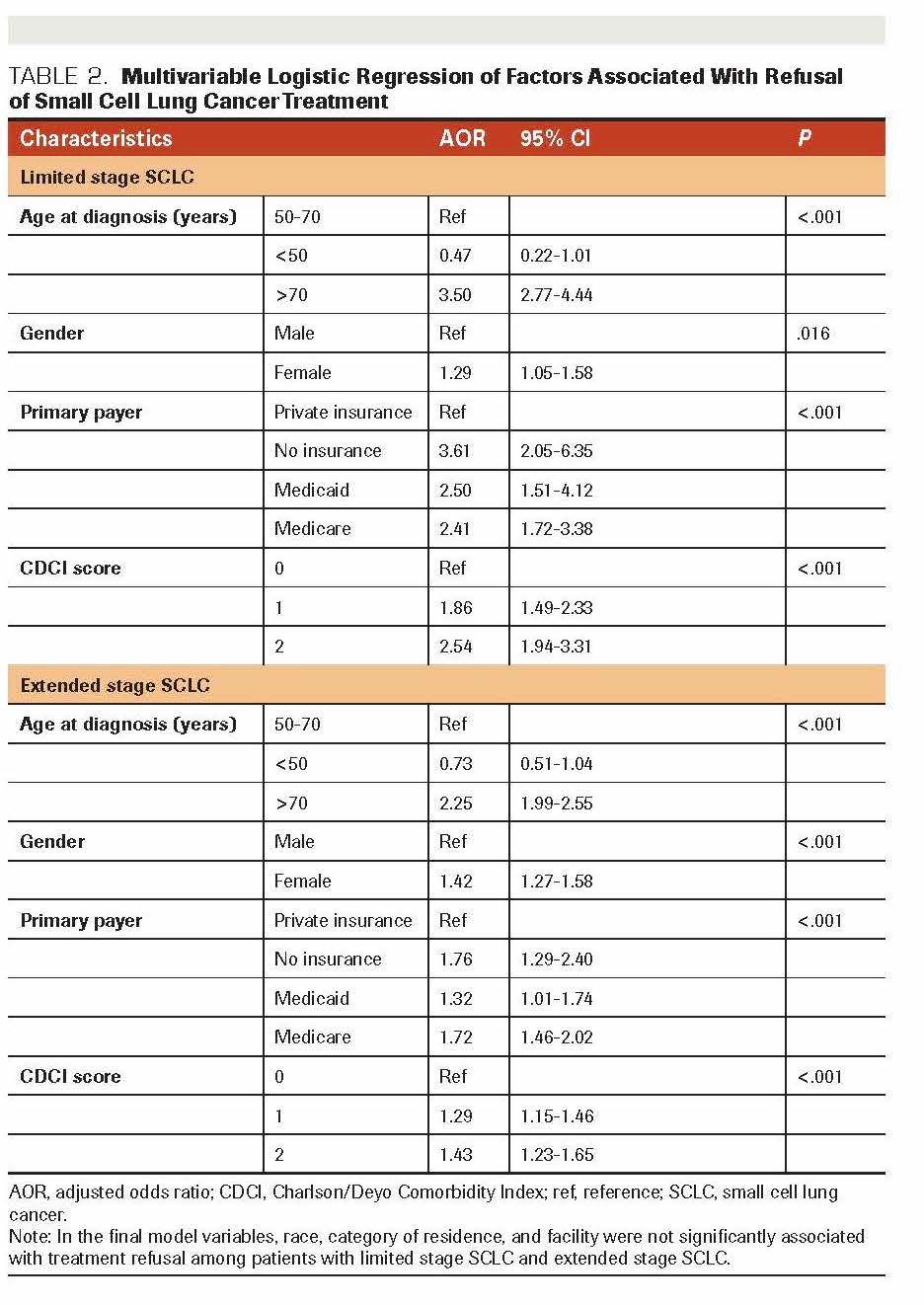
TABLE 3. Multivariable Cox Regression of Factors Associated With Survival of Patients With Small Cell Lung Cancer
Survival of SCLC Cases
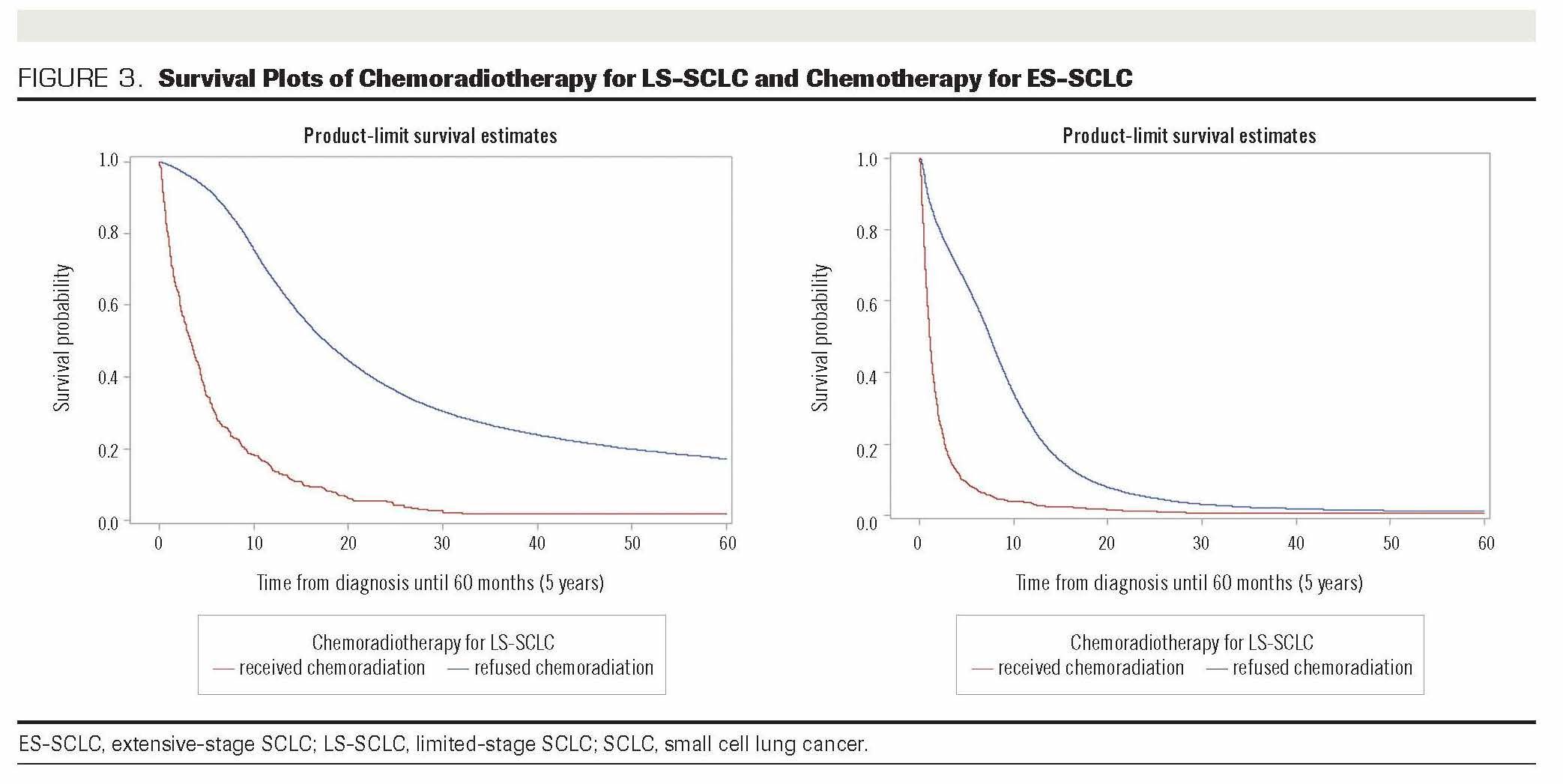
The overall median survival for all patients with SCLC included in the analysis was 9.4 months. Among patients with LS-SCLC who were recommended chemoradiotherapy, the median survival of patients who refused and received the treatment was 3 months and 18 months, respectively (P <.001) (Figure 3). For patients with ES-SCLC, median survival of those who refused chemotherapy was 1 month compared with 7.5 months for patients who received treatment (P < .001). Within 1 year following diagnosis (data not shown) among patients with LS-SCLC who were recommended chemoradiotherapy, 67% who received the treatment had survived, compared with 14% of those who declined. The 5-year survival of patients with LS-SCLC who received chemoradiotherapy was 17% compared with only 2% of those who refused (Figure 3). For ES-SCLC, the 1-year survival of patients who received the recommended chemotherapy was 25%, compared with 3% of those who refused.
We included age, gender, primary payer, category of residence, presence of comorbidity, facility, and treatment status in multivariable analyses using Cox PH regression. Results are presented in Table 3 and indicate that among patients with LS-SCLC who were recommended chemoradiotherapy, those who refused treatment had a higher risk of mortality than those who received treatment (HR, 4.96; 95% CI, 4.45-5.53). Similarly, among patients with ES-SCLC who were recommended chemotherapy, patients who refused treatment had a higher risk of mortality than those who received it (HR, 3.69; 95% CI, 3.48-3.92).
FIGURE 3. Survival Plots of Chemoradiotherapy for LS-SCLC and Chemotherapy for ES-SCLC
Discussion
Our study found that the majority of first-line treatments received by patients with SCLC in the database met national clinical practice guidelines, which include a combination of chemotherapy and radiation therapy for LS-SCLC and chemotherapy alone for ES-SCLC.5,15 Regarding survival among both the LSSCLC and ES-SCLC groups, patients who accepted recommended treatment had significantly higher survival than those who refused. The overall refusal of the recommended chemoradiotherapy among patients with LS-SCLC was 1.34%, and refusal of chemotherapy among those with ES-SCLC was 4.70%. These proportions are smaller than the overall treatment refusal reported by Ward et al, which was 9% from an analysis of 11 cancer facilities.16 Our analyses indicated that over time, there were increases in the proportions of treatment refusal of patients with both LS-SCLC and ES-SCLC. In particular, among patients with ES-SCLC, the refusal of treatment increased by 50% in 10 years. The fact that three-fourths of SCLC diagnoses are for ES-SCLC suggests that our findings on treatment refusal deserve special attention. Aizer et al indicate that compared with other cancers, refusal of treatment among patients with SCLC was more likely to result in death due to cancer.17
Of factors associated with treatment refusal, age at diagnosis was significantly associated with refusal of both chemoradiotherapy for LS-SCLC and chemotherapy for ES-SCLC. After adjustment for other characteristics, patients receiving a diagnosis at a younger age were less likely to refuse treatment compared with older patients. A meta-analysis among patients with LSSCLC suggested that due to the toxicity associated with chemoradiotherapy, the survival benefit from treatment was limited to younger patients.18
However, results of other studies have shown that both older and younger patients respond just as well to a combined modality therapy.19,20 Results of a recent study using NCDB data suggested that older patients who received combined modality therapy had a better overall survival than those who had chemotherapy alone.21 Younger patients are considered to have better performance status, fewer comorbid conditions, and increased life expectancy, which are all factors that support the decision to agree to cancer treatment.22 Older patients, on the other hand, have increased concerns regarding the tolerability of chemotherapy, treatment duration, and effectiveness and tend to have more comorbid conditions that affect the decision to decline the offered treatment.22,23 Analyses of the population and facility-based data in previous studies have shown a continuous increase in the proportion of patients with SCLC diagnosed who were aged more than 70 years.24-26 If more patients with SCLC are diagnosed at an older age, it may be reasonable to expect that the proportion of treatment refusal will continue to rise, due in part to the aforementioned array of concerns of older patients.
Regarding association with gender, previous studies have suggested an increased incidence of SCLC cases among women.27,28 Women with SCLC were also shown to have better survival than men,6,27 with gender differences in survival being linked to patients’ perceptions of cancer care.29,30 A recent study by Lee et al showed that more women than men chose to refuse active first-line therapy after their diagnosis of SCLC.31 These findings are in agreement with our results, which suggested that women were more likely to refuse the recommended chemoradiotherapy for LS-SCLC and chemotherapy for ES-SCLC. Refusal linked to gender may occur because women with advanced cancer tend to value quality of life more than just prolonged life.32 As also indicated in the results of other cancer studies, women seem to be more vulnerable to treatment toxicities than men.30 In addition, they were more likely than men to have planned more specific late-in-life activities and were therefore less likely to want, at such a time in their lives, to repeatedly visit medical facilities to complete their cancer treatment series.33 These perceptions may affect women’s decisions to decline recommended cancer treatment. Our findings suggest the need for future studies among women to investigate their perceptions of SCLC treatment and expectations regarding their cancer treatment.
Uninsured patients were 2 to 3 times more likely to decline chemoradiotherapy for LS-SCLC and to decline chemotherapy for ES-SCLC than those with private insurance. Results of other studies among patients with lung cancer cases have suggested that uninsured patients were more likely to refuse offered cancer treatments.34,35 In addition, Halpern et al suggested that the uninsured were more likely to be diagnosed with advanced-stage disease.35 The American Society of Clinical Oncology has raised concerns about inequities in cancer care due to insurance status.36 A study by Duh et al estimated that the average cost of intravenous chemotherapy for SCLC was $788 per visit or $9449 per treatment course (3 visits per cycle for 4 cycles).37 For uninsured patients, these costs may affect the decision to accept or decline recommended cancer treatment. Furthermore, any factor that delays treatment could result in a more advanced and aggressive disease. To ensure delivery of quality cancer treatment and optimize patient outcomes, it is important to identify and remove impediments related to the health payer.
Results of survival analysis in this study demonstrate that the 1-year survival of patients with LS-SCLC who received chemoradiotherapy was 5 times higher than those who refused. Among patients with ES-SCLC, those who received chemotherapy were 8 times more likely to survive for 1 year compared with those who refused recommended treatment. The results found by Lally et al and Behera et al, similar to those of our study, previously suggested better survival rates among patients with SCLC who received treatment than those who did not.24,38 Regardless of the benefit on survival of receiving treatment, patient autonomy and locus of control remain important aspects of care. Patient-provider communication and patient education are essential in decision-making to increase patient acceptance of standard recommended treatment.
To our knowledge, our study is the most comprehensive one that has analyzed treatment refusal among patients with SCLC using a national facility-based database. We specifically examined the treatment refusal of chemoradiotherapy for LS-SCLC and chemotherapy alone for ES-SCLC, as these have the strongest evidence bases as stage-specific recommended treatment modalities for patients with SCLC. Our findings are relevant for patient management and future studies. The main strengths of our study are in the use of comprehensive clinical information, including cancer stage and treatment recommendations, and the large sample size of SCLC cases included in our analyses. We used the database that includes approximately three-fourths of newly diagnosed cancer cases in the US population.9,10
The main limitations of our study are those seen with retrospective and database studies in general. Although the database covers a majority of cancer cases in the United States, about a quarter of patients with new diagnoses are not captured in the data.35 However, only marginal differences between NCDB and the SEER data have been acknowledged and should not meaningfully affect our findings.39 Our findings suggest that future studies should focus on factors that might contribute to treatment decisions: for example, patient–physician interactions, patient education regarding cancer treatment and care, and patient support networks.
Conclusions
Although the proportion of patients refusing treatment for SCLC is relatively low, the increase in treatment refusal over time is concerning. Older age at diagnosis, female gender, uninsured status, and comorbidities were associated with higher refusal of chemoradiotherapy among patients with LS-SCLC and with higher refusal of chemotherapy among those with ES-SCLC. It is important for health providers and policymakers to not only make good treatment recommendations but also consider the ways their recommendations are delivered to patients. Interventions that target factors associated with higher treatment refusal may increase acceptance of recommended treatment and ultimately improve patient survival. Due to recent advancements in immunotherapy treatment for SCLC, there may be changes in treatment refusal. There is a need for comparative study to assess any change in treatment refusal among patients with SCLC.
References:
1. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review 1975-2014. National Cancer Institute / Surveillance, Epidemiology, and End Results program. Updated April 2, 2018. Accessed January 29, 2021. https://seer.cancer.gov/csr/1975_2014/
2. Gaspar LE, McNamara EJ, Greer Gay E, et al. Small-cell lung cancer: prognostic factors and changing treatment over 15 years. Clin Lung Cancer. 2012;13(2):115-122. doi:10.1016/j.cllc.2011.05.008
3. Averett Byers L, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121(5):664-672. doi:10.1002/cncr.29098
4. Pietanza MC, Averett Byers L, Minna JD, Rudin CM. Small cell lung cancer: will recent progress lead to improved outcomes?. Clin Cancer Res. 2015;21(10):2244-2255. doi:10.1158/1078-0432.CCR-14-2958
5. Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e400S-e419S. doi:10.1378/chest.12-2363
6. Ganti AK, West WW, Zhen W. Current concepts in the management of small cell lung cancer. Indian J Med Res. 2013;137(6):1043-1051.
7. Parsons HM, Harlan LC, Stevens JL, Dansky Ullmann C. Treatment of small cell lung cancer in academic and community settings: factors associated with receiving standard therapy and survival. Cancer J. 2014;20(2):97-104. doi:10.1097/PPO.0000000000000039
8. Recalcitrant Cancer Research Act of 2012. Pub L No 112-176, 112 Stat 733. September 20, 2012. Accessed January 29, 2021. https://www.congress.gov/bill/112th-congress/house-bill/733
9. About the National Cancer Database. American College of Surgeons. Accessed January 12, 2021. https://www.facs.org/quality%20programs/cancer/ncdb/about
10. Lerro CC, Robbins AS, Phillips JL, Stewart AK. Comparison of cases captured in the National Cancer Data Base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20(6):1759-1765. doi:10.1245/s10434-013-2901-1
11. Carter BW, Glisson BS, Truong MT, Erasmus JJ. Small cell lung carcinoma: staging, imaging, and treatment considerations. Radiographics. 2014;34(6):1707-1721. doi:10.1148/rg.346140178
12. American College of Surgeons. National Cancer Data Base Participant Use Data File (PUF) Data Dictionary. Version: PUF 2014. Accessed January 12, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/ncdb/puf%20data%20dictionary%20version%20puf%202014.ashx
13. Esnaola NF, Gebregziabher M, Knott K, et al. Underuse of surgical resection for localized, non-small cell lung cancer among whites and African Americans in South Carolina. Ann Thorac Surg. 2008;86(1):220-226; discussion 227. doi:10.1016/j.athoracsur.2008.02.072
14. Ryoo JJ, Ordin DL, Antonio ALM, et al. Patient preference and contraindications in measuring quality of care: what do administrative data miss?. J Clin Oncol. 2013;31(21):2716-2723. doi:10.1200/JCO.2012.45.7473
15. NCCN. Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer, version 2.2021. Accessed January 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
16. Ward MM, Ullrich F, Matthews K, et al. Access to chemotherapy services by availability of local and visiting oncologists. J Oncol Pract. 2014;10(1):26-31. doi:10.1200/JOP.2013.001217
17. Aizer AA, Chen MH, Parekh A, et al. Refusal of curative radiation therapy and surgery among patients with cancer. Int J Radiat Oncol Biol Phys. 2014;89(4):756-764. doi:10.1016/j.ijrobp.2014.03.024
18. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327(23):1618-1624. doi:10.1056/NEJM199212033272302
19. Yuen AR, Zou G, Turrisi AT, et al. Similar outcome of elderly patients in Intergroup Trial 0096: cisplatin, etoposide, and thoracic radiotherapy administered once or twice daily in limited stage small cell lung carcinoma. Cancer. 2000;89(9):1953-1960. doi:10.1002/1097-0142(20001101)89:9<1953::aid-cncr11>3.3.co;2-y
20. Schild SE, Stella PJ, Brooks BJ, et al. Results of combined-modality therapy for limited-stage small cell lung carcinoma in the elderly. Cancer. 2005;103(11):2349-2354. doi:10.1002/cncr.21034
21. Corso CD, Rutter CE, Park HS, et al. Role of chemoradiotherapy in elderly patients with limited-stage small-cell lung cancer. J Clin Oncol. 2015;33(36):4240-4246. doi:10.1200/JCO.2015.62.4270
22. Ludbrook JJS, Truong PT, MacNeil MV, et al. Do age and comorbidity impact treatment allocation and outcomes in limited stage small-cell lung cancer? a community-based population analysis. Int J Radiat Oncol Biol Phys. 2003;55(5):1321-1330. doi:10.1016/s0360-3016(02)04576-5
23. Puts MTE, Tapscott B, Fitch M, et al. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treat Rev. 2015;41(2):197-215. doi:10.1016/j.ctrv.2014.12.010
24. Lally BE, Geiger AM, Urbanic JJ, et al. Trends in the outcomes for patients with limited stage small cell lung cancer: an analysis of the Surveillance, Epidemiology, and End Results database. Lung Cancer. 2009;64(2):226-231. doi:10.1016/j.lungcan.2008.08.010
25. Dores GM, Qubaiah O, Mody A, Ghabach B, Devesa SS. A population-based study of incidence and patient survival of small cell carcinoma in the United States, 1992-2010. BMC Cancer. 2015;15:185. doi:10.1186/s12885-015-1188-y
26. Schabath MB, Nguyen A, Wilson P, Sommerer KR, Thompson ZJ, Chiappori AA. Temporal trends from 1986 to 2008 in overall survival of small cell lung cancer patients. Lung Cancer. 2014;86(1):14-21. doi:10.1016/j.lungcan.2014.07.014
27. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol. 2006;24(28):4539-4544. doi:10.1200/JCO.2005.04.4859
28. Osann KE, Lowery JT, Schell MJ. Small cell lung cancer in women: risk associated with smoking, prior respiratory disease, and occupation. Lung Cancer. 2000;28(1):1-10. doi:10.1016/s0169-5002(99)00106-3
29. Johnson MF, Lin M, Mangalik S, Murphy DJ, Kramer AM. Patients’ perceptions of physicians’ recommendations for comfort care differ by patient age and gender. J Gen Intern Med. 2000;15(4):248-255. doi:10.1111/j.1525-1497.2000.07004.x
30. Fletcher K, Prigerson HG, Paulk E, et al. Gender differences in the evolution of illness understanding among patients with advanced cancer. J Support Oncol. 2013;11(3):126-132. doi:10.12788/j.suponc.0007
31. Lee K, Kloecker G, Pan J, Rai S, Dunlap NE. The integration of multimodality care for the treatment of small cell lung cancer in a rural population and its impact on survival. Am J Clin Oncol. 2015;38(5):448-456. doi:10.1097/COC.0b013e3182a5346d
32. Sharma RK, Prigerson HG, Penedo FJ, Maciejewski PK. Male-female patient differences in the association between end-of-life discussions and receipt of intensive care near death. Cancer. 2015;121(16):2814-2820. doi:10.1002/cncr.29417
33. Citrin DL, Bloom DL, Grutsch JF, Mortensen SJ, Lis CG. Beliefs and perceptions of women with newly diagnosed breast cancer who refused conventional treatment in favor of alternative therapies. Oncologist. 2012;17(5):607-612. doi:10.1634/theoncologist.2011-0468
34. Grant SR, Walker GV, Guadagnolo BA, Koshy M, Allen PK, Mahmood U. Variation in insurance status by patient demographics and tumor site among nonelderly adult patients with cancer. Cancer. 2015;121(12):2020-2028. doi:10.1002/cncr.29120
35. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222-231. doi:10.1016/S1470-2045(08)70032-9
36. American Society of Clinical Oncology. The state of cancer care in America, 2015: a report by the American Society of Clinical Oncology. J Oncol Pract. 2015;11(2):79-113. doi:10.1200/JOP.2015.003772
37. Duh MS, Reynolds Weiner J, Lefebvre P, Neary M, Skarin AT. Costs associated with intravenous chemotherapy administration in patients with small cell lung cancer: a retrospective claims database analysis. Curr Med Res Opin. 2008;24(4):967-974. doi:10.1185/030079908x280464
38. Behera M, Ragin C, Kim S, et al. Trends, predictors, and impact of systemic chemotherapy in small cell lung cancer patients between 1985 and 2005. Cancer. 2016;122(1):50-60. doi:10.1002/cncr.29674
39. Mettlin CJ, Menck HR, Winchester DP, Murphy GP. A comparison of breast, colorectal, lung, and prostate cancers reported to the National Cancer Data Base and the Surveillance, Epidemiology, and End Results Program. Cancer. 1997;79(10):2052-2061. doi:10.1002/(sici)1097-0142(19970515)79:10<2052::aid-cncr29>3.0.co;2-s
