Local Therapy for Breast Cancer in the Molecular Era: Relevant or Relic?
This article reviews the current status of local therapy for breast cancer and the likely impact of evolving molecular data on the present paradigm.
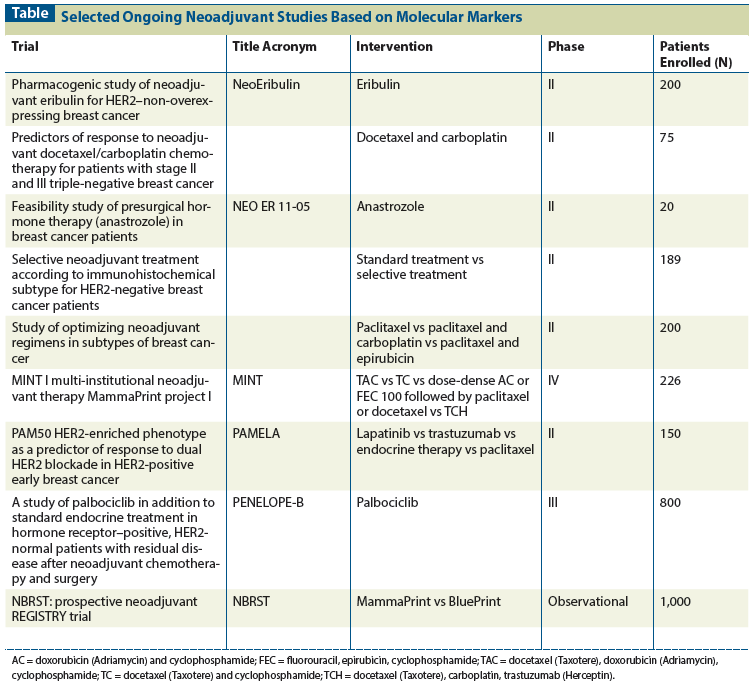
Table: Selected Ongoing Neoadjuvant Studies Based on Molecular Markers
Local therapy (surgery and radiation) is an essential component of breast cancer treatment. Yet, based on clinical trial results dating from the 1980s, the magnitude of local therapy interventions has been decreasing. Now, with the emergence of tailored systemic therapies, their increasing use in the neoadjuvant setting, and their high rates of pathologic complete response (pCR), the relevance of local therapy is being questioned. However, given our present inability to assess pathologic response without surgery, the low pCR rates in common subtypes of breast cancer, and the survival advantages with nodal radiation, local therapy remains not only relevant but crucial to a comprehensive breast cancer treatment plan. In the future, as gene profiling of individual patient tumors progresses and allows for precise tailoring of therapy, it is conceivable that surgery and/or radiation would not be required in some patients. However, the notion that brief, low-morbidity local therapy options, which synergize with prolonged and potentially morbid systemic regimens, would not be relevant for most patients is beyond the horizon at the moment.
Background
Local therapy (surgery with or without radiotherapy [RT]) forms the backbone of breast cancer treatment. Surgery was the original treatment for breast cancer, and RT was added around 1900, soon after the discovery of x-rays by Wilhelm Roentgen. These modalities remain the central pillars of all therapeutic plans for breast cancer patients. However, the magnitude of surgical interventions has decreased in recent decades, and breast conservation has replaced mastectomy for many patients, based on randomized trials showing equivalent survival.[1-5] Similarly, routine axillary dissection has given way to sentinel node biopsy for most patients.[6] On the other hand, the role of RT has not diminished; the appropriate use of adjuvant RT contributes to improved survival and remains an important component of current standards of care.
At the same time that the extent of surgery has been decreasing, local control has been increasing. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 trial (in which chemotherapy was used only for node-positive disease) showed a 14.3% in-breast tumor recurrence rate at 20 years[7] vs recurrence rates of 3.5% to 6.5% in more recent NSABP systemic therapy trials.[8] The reasons for this decrease are multifactorial and include changes in local management,[9] the addition of boost RT,[10] and improvements in systemic therapy, such as the introduction of aromatase inhibitors[11] and the addition of taxanes and anti–human epidermal growth factor receptor 2 (HER2) agents. These improvements can also be attributed to better local therapy, based on improvements in RT and imaging, as well as standardization of surgical techniques[12] and pathologic margin assessment.[13] At the moment, there is no question that adequate local therapy is crucial for local control, which in turn imparts a survival benefit[14] and improved quality of life. Therapy for local recurrences may entail removal of a breast or part of the chest wall, or nodal RT, each with the potential for symptomatic and functional loss, emotional distress, and added cost.
In addition, it has become clear that local control is also dependent on optimal systemic therapy, based on breast cancer subtype. The risk of local recurrence varies with the molecular subtype as determined by estrogen receptor (ER), progesterone receptor (PR), and HER2 status.[15] Large studies have demonstrated higher local recurrence rates according to breast cancer subtype, particularly in HER2-positive patients not treated with trastuzumab and triple-negative patients, with a trend toward higher local recurrence in patients with the luminal B subtype.[16,17] Other work suggests that, even within subtypes, there is remarkable heterogeneity among tumors; there are six different subgroups within the triple-negative subtype, and all have differing sensitivities to different chemotherapies.[18] With the advent of increasingly precise molecular profiling of breast cancer, it is likely that targeted systemic therapy will continue to improve.[19] How this will shift the balance between local and systemic therapeutic options is of considerable interest. Here we review the current status of local therapy for breast cancer and the likely impact of evolving molecular data on the present paradigm.
Current Guidelines: Why Local Therapy Remains Relevant
Surgical resection: the present landscape
Today, the treatment plan for most breast cancer patients begins with an assessment of the type of surgery required. For stage I and II breast cancer patients, breast conservation remains the standard of care: the tumor must be excised with microscopically free margins, and axillary nodes must be evaluated for pathologic evidence of tumor involvement. Mastectomy remains an alternative, although carved-in-stone indications for it continue to shrink. Thus, the pregnant patient, for whom mastectomy used to be the gold standard, can now be reasonably treated with neoadjuvant chemotherapy in the mid-trimester, and breast conservation following delivery can be offered if tumor characteristics allow.[20] Multicentricity, another long-standing indication for mastectomy, is also being reconsidered to determine whether breast conservation might be offered instead. The ongoing American College of Surgeons Oncology Group (ACOSOG) Z1071 trial, a single-arm prospective study, is assessing whether breast conservation is appropriate for selected patients with multicentric invasive breast cancer.[21] Radiation Therapy Oncology Group trial 1014 (see www.rtog.org for protocol information) is exploring the possibility of repeat breast conservation in selected patients with in-breast recurrence and prior RT. Across the board, surgical research on local therapy is focusing on strategies to reduce the burden of surgery on the breast cancer patient. At the same time, the rule of complete surgical excision of the primary tumor site and sufficient nodal evaluation to document pathologic nodal status stands intact.
Resection margin width
After 2 decades of shifting opinions regarding the desirable width of surgical margins for breast-conserving therapy, recent studies are relevant.[22,23] Based on a meta-analysis of 33 studies in women with stage I and II breast cancer (N = 28,162) by the Society of Surgical Oncology and the American Society for Radiation Oncology, there was no increase in local recurrence in studies where free margins were defined as no ink on tumor vs those requiring wider free margins. Correspondingly, a positive margin (ink on tumor) was associated with a twofold increase in the risk of ipsilateral breast tumor recurrence that was not negated by favorable biology, endocrine therapy, or radiation boost.[23] Covariates of endocrine therapy and median year of recruitment did not change the findings. These results suggest that, even as systemic therapy has improved, a free surgical margin, implying a minimal burden of residual disease, remains an important goal in local therapy and retains relevance to oncologic practice.[24] On a closer look at these data, there is a suggestion of better local control with wider margins in older studies, but this difference disappears in more recent studies, underscoring recent improvements-including those in systemic therapy-that may be contributing to better local control. This more parsimonious approach to tumor resection, however, does have the advantage of avoiding re-excision for close margins, thereby avoiding reoperation-associated morbidities and lessening patient distress and costs.
Surgery following neoadjuvant therapy
The most common indication for mastectomy has traditionally been a large tumor or an unfavorable tumor-to-breast-size ratio. In this setting, randomized trials have shown that breast conservation is feasible for many patients following preoperative chemotherapy, with or without anti-HER2 agents, as dictated by the tumor’s biologic profile.[25,26] This applies even for older women following neoadjuvant endocrine therapy with anastrozole or letrozole.[27,28] Despite the insertion of neoadjuvant systemic therapy into the initial treatment plans of stage I/II breast cancer patients, there is no evidence so far that the traditional components of local therapy can be eliminated. Thus, following a clinical or radiologic complete response to neoadjuvant systemic therapy, surgical excision of the tumor site remains standard of care.[12] In the NSABP B-18 trial, in which pre- and postoperative use of the current standard anthracycline regimen were compared, the complete clinical response rate was 36%, and only a quarter of these patients had a pathologic complete response (pCR).[29] When MRI was recently used to predict pCR, 12.5% of women demonstrating a complete radiologic response had pathologic evidence of residual disease upon tumor site resection.[30] Although pCR following systemic treatment is a predictor of locoregional control in NSABP trials,[29,31,32] and although current National Comprehensive Cancer Network (NCCN) guidelines (see www.nccn.org) support the use of neoadjuvant chemotherapy to aid in breast conservation, surgical resection and breast RT remain an integral part of the therapeutic strategy. Also, despite the downstaging of the axilla in 30% to 40% of women who present with biopsy-proven axillary nodal involvement,[21] surgical-pathologic axillary staging with sentinel node biopsy (using two tracers and harvesting of at least two sentinel nodes) remains necessary, to be followed by level I/II node dissection if sentinel nodes remain positive for tumor.
Is neoadjuvant systemic therapy reasonable prior to mastectomy?
In the absence of specific indications (locally advanced disease, a tumor-to-breast-size ratio that does not favor breast conservation in a woman who desires it), some recommend neoadjuvant systemic therapy as a means of evaluating tumor responsiveness to a particular regimen. This strategy has been tremendously useful in the evaluation of new therapies in clinical trials. Outside of clinical trials-where there is no proven follow-up therapy for the patient with a significant residual disease burden once a standard-of-care regimen has been delivered preoperatively-it is questionable. This is particularly true in women with triple-negative breast cancer. NCCN guidelines endorse the use of regimens recommended in the adjuvant setting for preoperative use in order to enable breast conservation, but they caution that the benefits of switching therapies following a limited response are not established. Overall, though, improving systemic therapy has clearly had an impact on the extent of surgical resection deemed necessary for optimal local control.
Postoperative radiotherapy
RT is an integral part of local therapy for breast cancer. The impact of RT on local control has been evident for decades, and data on a survival benefit are accumulating. The use of RT is part and parcel of breast-conserving therapy, and current guidelines allow breast conservation surgery only in patients who can undergo radiation.[12] The 20-year follow-up of the NSABP B-06 trial showed a 14.3% in-breast recurrence rate in women who received postoperative breast radiation vs 39.2% in those who did not.
These findings are echoed in essentially all trials that have tested breast-conserving surgery in early disease, with and without radiation. Further, they are well documented in the overview analysis of the Early Breast Cancer Trialists Collaborative Group (EBCTCG), where risk of any recurrence (locoregional or distant) at 10 years was reduced from 35% to 19%,[14,33] and the risk of breast cancer death was reduced by 3.8% in RT-treated women. There was a marked difference in the proportion of first recurrences that were locoregional: 25% in women who did not receive RT vs 8% in those who did. In high-risk women who received RT, there was a concomitant 7% reduction in the absolute risk of death. The EBCTCG analysis identified young age, grade, ER status, tamoxifen use, and extent of surgery as the factors that predict locoregional recurrence, although the extent of surgery parameter is difficult to interpret since the categories were lumpectomy vs sector resection, not categories based on microscopic margin status. In women with positive nodal disease, the 10-year recurrence risk was reduced from 63.7% to 42.5% with the addition of radiation, and the 15-year risk of breast cancer death was reduced from 51.3% to 42.8%.[14]
In the 1990s, RT was also shown to confer a survival benefit in women after mastectomy. Randomized clinical trials demonstrated a survival advantage and a reduction in recurrence when the chest wall and regional lymph nodes were radiated after mastectomy and axillary lymph node dissection in women with positive axillary disease.[34-38] Following mastectomy for node-positive breast cancer, the 10-year locoregional recurrence risk is markedly reduced by the use of RT (from 26% to 8.1%), as is the risk of any recurrence (from 63.5% to 51.9%) and of breast cancer death (from 66.4% to 58.3%). Thus, RT is increasingly recommended for all postmastectomy patients with involved axillary lymph nodes. Even RT in patients with one to three positive nodes, which was long controversial, can now be “strongly considered,” according to NCCN guidelines.[12] Nodal radiation is also increasingly recommended, based on initial results from the National Cancer Institute of Canada (NCIC) MA.20 trial.[39] This trial enrolled 1,832 women undergoing breast conservation; 85% had one to three positive axillary nodes and 91% received adjuvant endocrine therapy; there was sparse use of chemotherapy (5%). At a median follow-up of 62 months, the group receiving regional nodal RT experienced significant improvement in isolated locoregional disease-free survival, with a hazard ratio (HR) of 0.59 (P = .02). The absolute difference was modest (96.8% and 94.5%, for RT vs no RT, respectively). However, distant disease-free survival was also reduced with the use of regional nodal irradiation (5-year risk: 92.4% and 87.0%, respectively; HR = 0.64; P = .002), as was overall survival (5-year risk: 92.3% and 90.7%, respectively; HR = 0.76; P = .07). The potential survival value of nodal RT is further confirmed by results from the European Organisation for Research and Treatment of Cancer (EORTC) 22922 trial, which assessed the value of irradiation to the internal mammary chain, as well as to the breast/chest wall and other nodal fields.[40] Many patients in this trial had relatively early disease (60% had pT1 tumors and 40% were pN0), and again there was a modest improvement in locoregional control. There was also a 3.7% absolute improvement in distant recurrence risk and a 3% absolute improvement in survival. Finally, the AMA-ROS trial (EORTC 22023) compared axillary dissection with axillary RT in women with a positive sentinel node biopsy and found equivalent outcomes, although the noninferiority design was compromised by a lower than expected number of events.[41]
Thus, the current standard of care is to radiate regional lymph nodes when four or more nodes are positive and to strongly consider radiation if one to three nodes are positive.[12] This latter indication for nodal RT is likely to be further consolidated once we have final results from these recent trials (NCIC MA.20, EORTC 22922, and AMAROS). Thus, accumulating evidence for a small but significant absolute improvement in all cancer outcomes appears to be extending-not decreasing-the indications and the extent of RT for breast cancer patients.
Might Local Therapy Become a Relic in the Future?
The major impetus for this question comes from the increasing success of systemic therapy, particularly when it is directed to specific therapeutic targets. Could breast cancer therapy, at some point, consist only of systemic agents, with no need for local interventions such as surgery or RT? The question began with the laboratory work of a surgeon, Dr. Bernard Fisher, and his colleagues,[42] and culminated in the concept of neoadjuvant therapy for breast cancer and the resultant clinical trial results of the NSABP and other groups. Before we discuss these findings, we should define the conditions that would have to be met for local therapy to become a relic (ie, “a surviving memorial of something past”). These would include the ability to select individual systemic therapy regimens, based on patient characteristics and molecular profiling, that have a very high likelihood of inducing a pCR; the ability to assess the primary site response with certainty using imaging and core needle biopsies; and for women with residual disease, the ability to offer effective second- and third-line regimens that will ablate disease at the primary site and achieve locoregional control similar to that achieved with local therapy.
Historical perspective
It is useful to review the evolution of data on the impact of systemic therapy on local control. Through the 1970s and 1980s, the role of adjuvant postoperative chemotherapy for women with node-positive breast cancer was established through clinical trials in the United States and Europe.[43,44] Local control as a function of chemotherapy use was not an initial focus of these studies, but by the 1980s, local outcomes were being recorded and reported. NSABP B-13 randomly assigned women with ER-negative, node-negative disease to no treatment or treatment with methotrexate and fluorouracil; the 10-year local recurrence rate was 13.4% without systemic treatment vs 2.6% with systemic treatment.[32] Similarly, the NSABP B-14 trial evaluated women with ER-positive, node-negative breast cancer randomized to receive either placebo or tamoxifen and showed a decrease in local recurrence at 10 years, from 14.7% with placebo to 4.3% with tamoxifen.[31] These and other data made it clear that systemic regimens that improved survival were also improving local control.
Neoadjuvant systemic therapy
The next development was the advent of neoadjuvant chemotherapy, and it is important to remember that the expectation from early neoadjuvant trials was that preoperative chemotherapy would lead to improved survival. This did not transpire. However, it became clear that neoadjuvant therapy would be a useful approach for women with tumors large enough to require mastectomy who desired breast conservation. The quality of subsequent local control was obviously of significant interest. A second, and perhaps more important, emerging concept was the value of pCR as an indicator of tumor chemosensitivity and as a predictor of overall survival; we will presently discuss this further. With regard to local control, pCR appears to predict benefit. A recent pooled analysis of NSABP B-18 and B-27 data examined local recurrence after 10 years in women who underwent surgery (either breast-conserving therapy or mastectomy) after neoadjuvant chemotherapy.[29] In women receiving breast-conserving treatment, the strongest predictors of local recurrence were age, clinical nodal status before neoadjuvant chemotherapy, and nodal/tumor response after neoadjuvant therapy. For women who underwent mastectomy, clinical tumor size, clinical nodal status, and nodal/tumor response after neoadjuvant chemotherapy were the independent predictors of local recurrence. These results also suggested that the impacts of age, clinical tumor size, and clinical nodal status on the absolute locoregional recurrence rates are low if a patient achieves a pCR in the breast with pathologically negative axillary nodes. Thus, response to therapy is a major determinant of both overall survival and local control. These analyses further demonstrated that, for most patients, systemic therapy’s contribution to survival and its contribution to local control are closely aligned. Preoperative regimens that result in primary site pCR predict both improved survival and better local control, and postoperative regimens that improve survival improve local control.
Molecular profiles and local control
The importance of hormone receptor expression in breast cancer prognosis and prediction of response to endocrine therapy has long been recognized, as has the fact that not all hormone receptor–positive tumors are endocrine-responsive-and there is clearly an aggressive ER-positive subset. Similarly, the value of HER2 amplification as a prognostic factor was documented in the mid-1980s.[45] However, the crystallization of these important biologic features into what we now consider the major biologic subtypes of breast cancer did not occur until gene array studies were performed in patients with known disease outcomes.[46] Over the past decade or so, biologic subtypes have become an integral part of describing the disease and determining the plan for medical therapy. The impact of these subtypes on local control is also becoming increasingly clear.[47-49] Based on receptor status, proliferative rate, and HER2 amplification, four major subtypes are clinically relevant: luminal A (ER/PR-positive, HER2-negative, low proliferation), luminal B (ER-positive, PR-negative or PR-low, HER2-negative, high proliferation), HER2 (HER2-positive), and triple-negative (ER/PR/HER2-negative).[50-52] The luminal B and luminal HER2 subtypes have the highest incidence of lymph node positivity, and triple-negative tumors are associated with larger size.[53] Several retrospective analyses have pointed to breast cancer subtype as a determinant of local recurrence risk, with the lowest risk for luminal A tumors and the highest risk for triple-negative tumors.[15-17] However, age and other variables still need to be factored into the equation.[16]
Other parameters of tumor biology that predict response to systemic therapy may also predict local control, as reflected in data on the 21-gene recurrence score (Oncotype DX).[54] This molecular profile was designed to predict the likelihood of distant metastasis in ER-positive, node-negative patients who received tamoxifen[55] but might also benefit from added chemotherapy. Women with high recurrence scores who were treated with chemotherapy and tamoxifen reduced their local recurrence rate to 7.8%, compared with 18.4% with no chemotherapy. Interestingly, there was a lower local recurrence rate in mastectomy patients than in breast conservation patients, with a significant interaction (P = .036) between recurrence score group and type of surgical treatment-raising the possibility that the recurrence score may also provide information about radiation resistance. Similarly, a 70-gene expression profile, MammaPrint, is stratifying patients into low and high risk for the development of distant metastases.[56] The MINDACT trial is currently comparing MammaPrint with traditional criteria in selecting node-positive women for adjuvant chemotherapy.[57] While results are not yet clear, these studies highlight the use of multigene expression systems and the importance of tumor biology for prognosis. Additional signatures that assess radiation sensitivity of breast cancer have been evaluated, with encouraging results, but they are not yet clinically useful.[49]
The HER2 model of targeted therapy
The most relevant current therapeutic target for this discussion is the HER2 protein. The targeting of hormone receptors, although important for optimal local control and survival, has not so far yielded pCR rates that could alter the need for local therapy (although neoadjuvant endocrine agents may reduce the extent of surgery required).[27] The success of HER2-targeted agents such as trastuzumab[58-60] serves to illustrate the added value of targeted therapy for local control. In the first phase III trials of adjuvant chemotherapy with or without trastuzumab, the number of locoregional recurrences was cut roughly in half by the addition of trastuzumab.[59] In a 3-year retrospective study, adjuvant trastuzumab therapy decreased local recurrence in HER2-positive, node-negative breast cancer patients who chose breast conservation from 10% to 1%.[61]
Benefits from trastuzumab have led to the development of additional anti-HER2 agents, and their development exemplifies the model in which new regimens are tested in a neoadjuvant setting before they are tested in a large randomized adjuvant trial. For example, the Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimization (NeoALTTO) trial showed improved benefit for neoadjuvant trastuzumab when combined with lapatinib. Dual anti-HER2 therapy combined with paclitaxel yielded a pCR rate of 51.3%; in contrast, the pCR rate was 24.7% for lapatinib and paclitaxel, and 29.5% for trastuzumab and paclitaxel.[62] As expected, those who achieved a pCR had a better outcome than those who did not-demonstrating that response to neoadjuvant trastuzumab therapy is the strongest predictor of recurrence and survival in HER2-positive cancers.[63]
These results were expected to be confirmed in the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO) trial. ALTTO assessed dual HER2-directed therapy (trastuzumab and lapatinib) vs trastuzumab alone or lapatinib alone in combination with chemotherapy in the adjuvant setting (N = 8,381). However, as reported at the 2014 American Society of Clinical Oncology meeting, the results were essentially null, with the trastuzumab-alone arm demonstrating survival that was equivalent to the trastuzumab-and-lapatinib arm.[64] Although the planned number of events was not reached, the data safety monitoring board concluded that the lapatinib-alone arm was unlikely to meet the prespecified criteria for the demonstration of noninferiority to the trastuzumab-alone arm with respect to disease-free survival, and the trial was closed.[65] Since then, much ink has been spilled analyzing and discussing these discrepant findings. It is more than likely that those women with tumors responsive to dual HER2 blockade in ALTTO benefited similarly to women in NeoALTTO, but they did not emerge as a distinct group (those achieving pCR at the primary site and excellent survival). As a result, lapatinib is not recommended for adjuvant therapy.
The same investigative model has been used for pertuzumab, the second HER2 antibody to reach clinical trials. Pertuzumab binds to a different epitope of HER2 and was shown to improve survival in the metastatic setting when combined with trastuzumab.[66] The addition of platinum agents to trastuzumab and pertuzumab also produced added benefit in the NeoSphere and TRYPHAENA trials. NeoSphere randomized patients with new operable, locally advanced, or inflammatory breast cancer to permutations of docetaxel with single or dual HER2 blockade (trastuzumab alone, pertuzumab alone, or both) vs dual blockade alone. Women who received all three drugs had a pCR of 45.8% vs a pCR of 29% in women who received dual blockade alone.[67] The phase II TRYPHAENA trial evaluated neoadjuvant trastuzumab and pertuzumab in combination with anthracycline- or carboplatin-based chemotherapy in women with nonmetastatic HER2-positive cancer.[68] Patients were randomized to receive neoadjuvant combinations of dual HER2 blockade plus fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel with or without carboplatin. All arms of this study performed well but the best pCR rate-66.2%-was observed in the pertuzumab, trastuzumab, docetaxel, and carboplatin group. These findings led to US Food and Drug Administration approval of pertuzumab for use in the neoadjuvant setting, and NCCN guidelines now endorse the use of adjuvant pertuzumab in patients with HER2-positive tumors when it is not used preoperatively. The APHINITY trial is assessing pertuzumab in the adjuvant setting. It has completed accrual of 4,800 patients with stage I–III HER2-positive breast cancer who have been randomly assigned to receive standard chemotherapy (nonanthracycline- or anthracycline-based) concurrent with pertuzumab/trastuzumab or to receive standard chemotherapy concurrent with trastuzumab alone.
If the results of APHINITY fit the model in which neoadjuvant success equals adjuvant success, they will provide a significant impetus for the testing of additional new agents in the neoadjuvant setting and for the routine use of neoadjuvant systemic therapy in all women who are likely to need a specific regimen postoperatively. Should this come to pass, we could start to think of surgical treatment as “adjuvant,” but as long as thorough pathologic assessment of the entire tumor bed is required, surgery will still be necessary.
Imaging of response
There is currently no replacement for surgical-pathologic evaluation of the primary tumor site and nodes to determine response to therapy. The accuracy of MRI, despite improvements in technology, hovers around 70%.[30,69,70] If imaging and concomitant imaging-guided biopsy techniques progress to the point where patients with pCR can be reliably identified, patients with a high likelihood of pCR (or almost pCR) could be spared surgery, but it is not conceivable at the moment that they would also be spared RT.
Switching regimens in patients with residual disease
The next step in the movement toward systemic therapy potentially replacing local therapy will be the development of effective second-line systemic regimens for women with residual disease following primary systemic therapy. So far, there has been little success with “switching” strategies[71]; however, ongoing studies are testing a range of strategies, including immunotherapy, for women with a high residual disease burden following neoadjuvant chemotherapy (www.clinicaltrials.gov). A great deal of progress is required-not only in systemic therapy regimens, but also in imaging assessment of disease burden-before there is even a possibility of abandoning surgery. The elimination of RT is not on the horizon at present.
Conclusions
Local therapy alone can cure some patients. Surgical resection is the simplest, quickest way to decrease and even (in some patients) eliminate tumor volume at the primary site. In women without micrometastatic disease at distant sites, it is a curative intervention, particularly when followed by RT. Studies of molecular profiling of tumors that allow the optimal selection of primary and secondary systemic therapy strategies should also generate data that enable the selection of women who do not need systemic therapy and who have an excellent chance for cure with local therapy alone or in combination with endocrine therapy. For these patients, local therapy will never be a relic.
Finally, it is important to remember that multimodality therapy resulted from the realization that the three main treatments (surgery, RT, systemic therapy) interact in a synergistic fashion. Although the importance of local therapy may wane as the efficacy of systemic therapy increases[72]-and present trends certainly suggest that systemic therapy may possibly become the “primary” therapy for many patients-the notion that local therapy will not be required for most patients is at present a thought experiment.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227-32.
2. Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567-75.
3. van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organisation for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143-50.
4. Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98:697-702.
5. Polgar C, Fodor J, Major T, et al. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol. 2013;108:197-202.
6. Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927-33.
7. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-41.
8. Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466-73.
9. Vicini FA, Eberlein TJ, Connolly JL, et al. The optimal extent of resection for patients with stages I or II breast cancer treated with conservative surgery and radiotherapy. Ann Surg. 1991;214:200-4; discussion 204-5.
10. Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513-20.
11. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687-717.
12. Theriault RL, Carlson RW, Allred C, et al. Breast cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:753-60; quiz 61.
13. Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer-bigger is not better. N Engl J Med. 2012;367:79-82.
14. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-106.
15. Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684-91.
16. Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885-91.
17. Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373-8.
18. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750-67.
19. Daemen A, Griffith OL, Heiser LM, et al. Modeling precision treatment of breast cancer. Genome Biol. 2013;14:R110.
20. Triunfo S, Scambia G. Cancer in pregnancy: diagnosis, treatment and neonatal outcome. Minerva Ginecol. 2014;66:325-34.
21. Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455-61.
22. Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2014;21:717-30.
23. Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys. 2014;88:553-64.
24. Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46:3219-32.
25. Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672-85.
26. Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676-85.
27. Ellis MJ, Coop A, Singh B, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19:3808-16.
28. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23:5108-16.
29. Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30:3960-6.
30. Ko ES, Han BK, Kim RB, et al. Analysis of factors that influence the accuracy of magnetic resonance imaging for predicting response after neoadjuvant chemotherapy in locally advanced breast cancer. Ann Surg Oncol. 2013;20:2562-8.
31. Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529-42.
32. Fisher B, Dignam J, Mamounas EP, et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors: eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil. J Clin Oncol. 1996;14:1982-92.
33. Early Breast Cancer Trialists’ Collaborative Group; Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707-16.
34. Hellman S. Stopping metastases at their source. N Engl J Med. 1997;337:996-7.
35. Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b trial. N Engl J Med. 1997;337:949-55.
36. Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641-8.
37. Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116-26.
38. Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539-69.
39. Whelan TJ, Olivotto I, Ackerman I, et al. NCIC-CTG MA.20: an intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol. 2011;29(suppl):abstr LBA1003.
40. Poortmans PSH, Kirkove C, Budach V, et al. Irradiation of the internal mammary and medial supraclavicular lymph nodes in stage I to III breast cancer: 10 years results of the EORTC radiation oncology and breast cancer groups phase III trial 22922/10925. Eur J Cancer. 2013;47(suppl 2):abstr BA2.
41. Donker M, Straver ME, van Tienhoven G, et al. Comparison of the sentinel node procedure between patients with multifocal and unifocal breast cancer in the EORTC 10981-22023 AMAROS trial: identification rate and nodal outcome. Eur J Cancer. 2013;49:2093-100.
42. Fisher B. Laboratory and clinical research in breast cancer-a personal adventure: the David A. Karnofsky memorial lecture. Cancer Res. 1980;40:3863-74.
43. Bonadonna G, Brusamolino E, Valagussa P, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294:405-10.
44. Wolmark N, Fisher B. Adjuvant chemotherapy in stage II breast cancer: a brief overview of the NSABP clinical trials. World J Surg. 1985;9:699-706.
45. Cicenas J, Urban P, Kung W, et al. Phosphorylation of tyrosine 1248-ERBB2 measured by chemiluminescence-linked immunoassay is an independent predictor of poor prognosis in primary breast cancer patients. Eur J Cancer. 2006;42:636-45.
46. Kao KJ, Chang KM, Hsu HC, Huang AT. Correlation of microarray-based breast cancer molecular subtypes and clinical outcomes: implications for treatment optimization. BMC Cancer. 2011;11:143.
47. Perou CM, Parker JS, Prat A, et al. Clinical implementation of the intrinsic subtypes of breast cancer. Lancet Oncol. 2010;11:718-9; author reply 20-1.
48. Miyamoto DT, Harris JR. Molecular predictors of local tumor control in early-stage breast cancer. Semin Radiat Oncol. 2011;21:35-42.
49. Kreike B, Halfwerk H, Armstrong N, et al. Local recurrence after breast-conserving therapy in relation to gene expression patterns in a large series of patients. Clin Cancer Res. 2009;15:4181-90.
50. Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736-47.
51. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869-74.
52. Prat A, Parker JS, Fan C, Perou CM. PAM50 assay and the three-gene model for identifying the major and clinically relevant molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;135:301-6.
53. Liao GS, Chou YC, Hsu HM, et al. The prognostic value of lymph node status among breast cancer subtypes. Am J Surg. 2014 Aug 5. [Epub ahead of print]
54. Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677-83.
55. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726-34.
56. Mook S, Knauer M, Bueno-de-Mesquita JM, et al. Metastatic potential of T1 breast cancer can be predicted by the 70-gene MammaPrint signature. Ann Surg Oncol. 2010;17:1406-13.
57. Cardoso F, Piccart-Gebhart M, Van’t Veer L, et al. The MINDACT trial: the first prospective clinical validation of a genomic tool. Mol Oncol. 2007;1:246-51.
58. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-72.
59. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-84.
60. Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29-36.
61. Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer. 2012;118:1982-8.
62. de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15:1137-46.
63. Kim MM, Allen P, Gonzalez-Angulo AM, et al. Pathologic complete response to neoadjuvant chemotherapy with trastuzumab predicts for improved survival in women with HER2-overexpressing breast cancer. Ann Oncol. 2013;24:1999-2004.
64. McCullough AE, Dell’orto P, Reinholz MM, et al. Central pathology laboratory review of HER2 and ER in early breast cancer: an ALTTO trial [BIG 2-06/NCCTG N063D (Alliance)] ring study. Breast Cancer Res Treat. 2014;143:485-92.
65. Hutchinson L. Breast cancer: ALTTO: wake-up call for setting up clinical trials. Nat Rev Clin Oncol. 2013;10:121.
66. Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461-71.
67. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25-32.
68. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24:2278-84.
69. Marinovich ML, Sardanelli F, Ciatto S, et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast. 2012;21:669-77.
70. De Los Santos JF, Cantor A, Amos KD, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119:1776-83.
71. von Minckwitz G, Kummel S, Vogel P, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst. 2008;100:542-51.
72. Punglia RS, Morrow M, Winer EP, Harris JR. Local therapy and survival in breast cancer. N Engl J Med. 2007;356:2399-405.