The Evolution of Liver-Directed Treatments for Hepatic Colorectal Metastases
This article will review the current practice of hepatic resection for colorectal liver metastases, including the possibility of combined resection of hepatic metastases at the time of resection of the primary cancer.
Figure 1: Patients With Extensive Hepatic Colorectal Metastases Can Be Effectively Treated
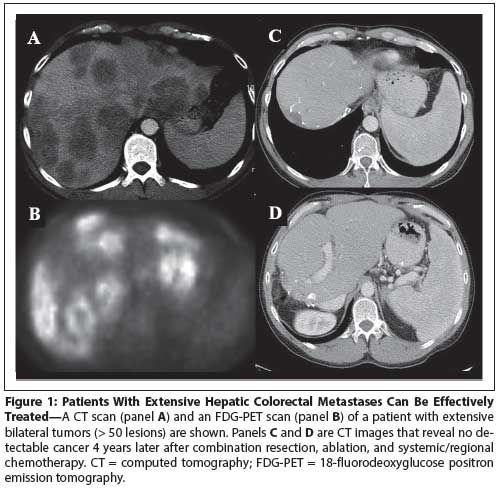
Figure 2: Irreversible Electroporation (IRE)
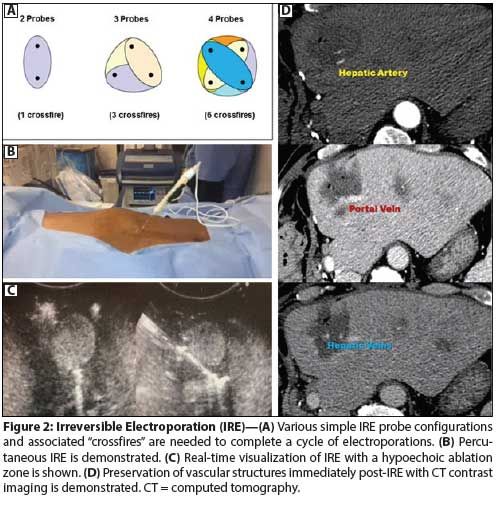
Figure 3: Algorithm of Treatment for Patients With Synchronous Hepatic Colorectal Metastases
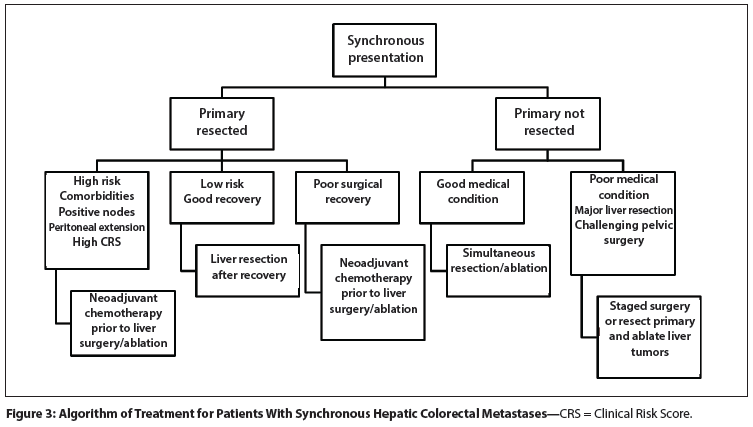
Figure 4: Algorithm of Treatment for Patients With Metachronous Hepatic Colorectal Metastases
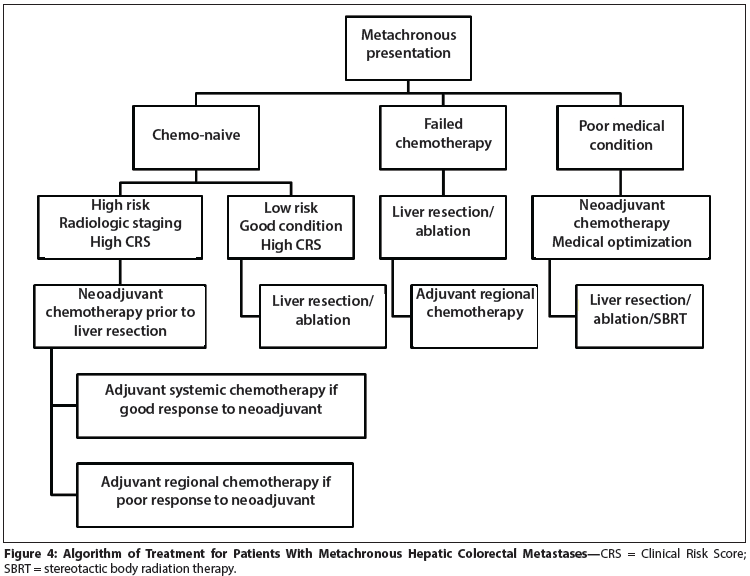
Table: 1 Forms of Liver-Directed Therapies
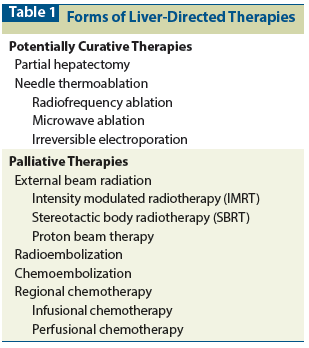
Table 2: Results of Liver Resection for Colorectal Metastases
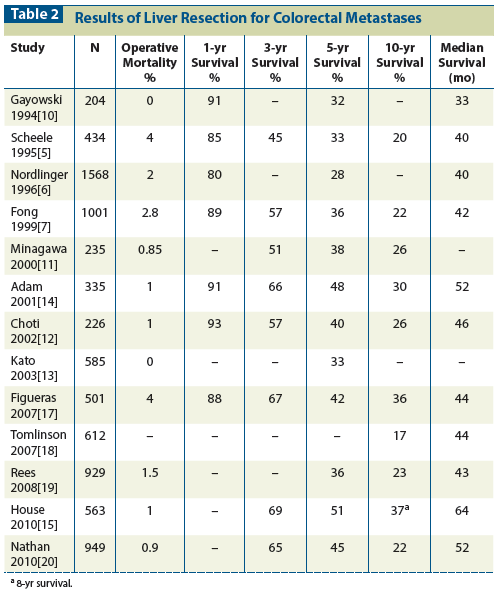
Table 3: Prognostic Variables for Hepatic Colorectal Metastases
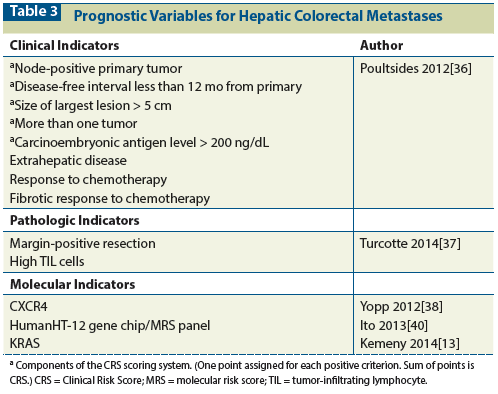
Table 4: Results of Downstaging Chemotherapy
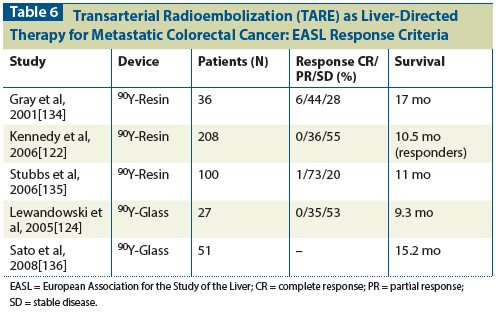
Table 5: Results of the Prospective Trials of SBRT for Liver Metastases
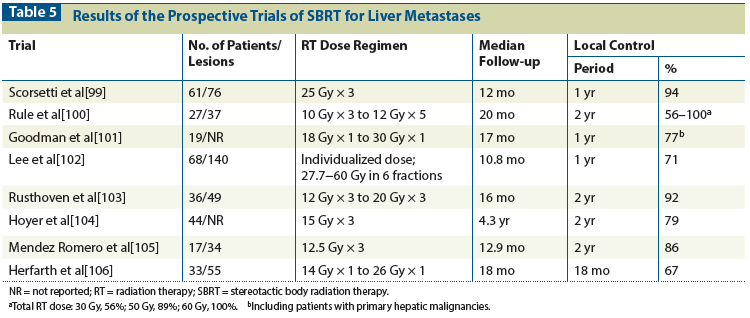
Table 6: Transarterial Radioembolization (TARE) as Liver-Directed Therapy for Metastatic Colorectal Cancer: EASL Response Criteria
The liver is the most common site of metastases from colorectal cancer. Over the past 3 decades, surgical resection has proved to be the most effective and potentially curative therapy for such metastases. This article will review the current practice of hepatic resection for colorectal liver metastases, including the possibility of combined resection of hepatic metastases at the time of resection of the primary cancer. Effective use of neoadjuvant and adjuvant chemotherapy has further expanded the pool of treatable patients. Most recently, ablative therapies based on needle-delivered thermoablation or radiation therapy have become additional weapons for effective treatment. The relative roles and combined use of these local therapies will be highlighted in this article. Overall, the recent combined advances in surgery, radiation therapy, ablative therapy, and chemotherapy have provided more patients with a chance for long-term survival.
Introduction
The liver has long been recognized as the most common site of systemic metastases from colorectal cancer. The reason for this is anatomic, since all venous blood from the intestines drains through the portal circulation to the liver. Acting as a filter, the liver prevents tumor cells from passing further. Most tumor cells arriving at the liver do not implant and develop the vasculature needed to survive.[1] Thus, even if millions of tumor cells enter the circulation and pass to the liver, only limited numbers of liver tumors may become clinically apparent.
Natural History of Colorectal Cancer Liver Metastases
In nearly one-half of patients in whom colorectal cancer is diagnosed, liver metastases will be found at some point during the disease. When untreated, patients with liver metastases have a median survival of 6 to 9 months.[2] Even with the best chemotherapy, the median survival of patients with unresectable disease is 18 to 23 months.[3,4] When the disease in the liver is unresectable or unresected, the liver site will be the cause of death in the majority (> 70%) of patients.[2] Thus, the hepatic site of disease dominates the clinical picture in most cases, explaining the large number of liver-directed therapies that have been developed and found effective in extending survival for patients with hepatic colorectal metastases (Table 1).
Partial Hepatectomy for Colorectal Liver Metastases
Encouraged by autopsy studies demonstrating that metastatic disease may be confined to the liver and by radiologic documentation of disease confined to the liver in many cases, surgeons increasingly began to resect hepatic colorectal metastases.[5-7] A large body of data has accumulated during the past decades proving that surgical resection is safe and effective therapy (Table 2).[5-20] In general, operative mortality at major centers is less than 5%, and 5-year survival can be achieved in more than one-third of patients. An interesting study with complete 25-year follow-up of patients treated by liver resection by one of the pioneers in the field has demonstrated that surgery alone can provide a cure in approximately 20% of patients.[21] Patients also recover quickly and return to normal life.[22] These results can be accomplished during short hospital stays of 5 to 10 days.[11-14] Because of these data, liver resection has become the standard of care for patients with hepatic colorectal metastases.
Patient selection for hepatectomy: staging of disease
In the 1980s, indications for liver resection for metastatic disease were medically fit patients with limited liver disease (generally, solitary lesions or fewer than four lesions in the same lobe of the liver). Thus, during the infancy of the field of hepatectomy for colorectal liver metastases, it was estimated that fewer than 10% of patients were candidates for surgery.
As safety and long-term cancer-related outcomes have improved, medical and oncologic indications have increasingly broadened. Advanced age is no longer a complete contraindication, and hepatectomies are now routinely performed in patients in their 80s.[23,24] Compensated medical comorbidities are also no longer a complete contraindication. Aggressive surgery is now considered for patients with extensive disease, including synchronous disease, bilobar disease, and numerous nodules (Figure 1).[25] Of interest, the overall survival of patients at major institutions has not worsened despite the expanding medical and oncologic indications for surgery. It is estimated that more than 50% of patients with hepatic colorectal metastases are now candidates for hepatectomy.
Need for Useful Clinical Staging Criteria
All patients with metastatic colorectal cancer in the liver are considered stage IV by the American Joint Committee on Cancer criteria. The population of patients offered hepatectomy for colorectal metastases is clearly quite heterogeneous. Investigators have thus been trying to devise clinical staging criteria for this population to better assist in choosing patients for surgery, adjuvant therapies, and trials, as well as to compare data from various institutions.
Building on previous trials, two large patient studies conceived very similar scoring systems for staging patients with hepatic colorectal metastases. Both systems used clinical variables related to the primary cancer and the liver metastases.[6,7] The five elements common to both systems have been popularized as the Clinical Risk Score (CRS) (Table 3)[7]: (1) nodal metastases from the primary cancer,[26] (2) short disease-free interval,[27-30] (3) size of the largest liver tumor,[27,31] (4) more than one liver metastasis,[27,30] and (5) high carcinoembryonic antigen level.[6,27]
The simplicity of the CRS has led to its widespread use. Investigators from many nations have independently verified this scoring system.[32,33] The CRS also predicts the prognosis of patients who undergo resection or ablation. In addition, it has been used to determine the extent of the preoperative diagnostic work-up needed to optimize yield while minimizing cost.[34,35]
Recently, investigators have attempted to improve on the CRS by adding clinical parameters such as response to chemotherapy or, more specifically, fibrotic response to chemotherapy[36]; immune assay results such as high tumor-infiltrating lymphocyte levels[37]; molecular tests such as those for CXCR4,[38] KRAS, vascular endothelial growth factor, or epidermal growth factor receptor[39]; or a panel of biomarkers.[40] These have shown some additional discriminating effect. However, until these molecular tests are employed universally in the analysis of tumors, they will be used mainly in tertiary centers and be of primarily academic interest.
Chemotherapy
We will only briefly summarize chemotherapy as it pertains to the perioperative treatment of patients with hepatic colorectal metastases. Recent years have seen an increase in the number and effectiveness of chemotherapeutic and biologic regimens for colorectal cancer. Nevertheless, few patients are ever cured with chemotherapy and/or biologic therapies alone.[41,42] Median survival for patients treated only with chemotherapy is approximately 18.6 months with FOLFIRI (leucovorin [LV], fluorouracil, and irinotecan),[4] 19.9 months with cetuximab-FOLFIRI,[4] and 19.7 months with FOLFOX4 (LV, ï¬uorouracil, and oxaliplatin).[3] Even patients with the most favorable KRAS wild-type tumors who were treated with panitumumab-FOLFOX4 had a median survival of less than 24 months, with few 5-year survivors.[3] Thus, the use of chemotherapy alone in patients with metastatic colorectal cancer is regarded as a palliative intervention.
Adjuvant systemic chemotherapy
Six recent studies have examined the role of adjuvant chemotherapy after liver resection for metastatic colorectal cancer. In a trial that randomized 173 patients to receive either adjuvant ï¬uorouracil (5-FU) and LV or no chemotherapy after resection,[43,44] a significant improvement in recurrence-free survival was found with adjuvant chemotherapy (34% vs 27%; P < .03). However, because of the small sample size, not surprisingly, the difference in overall survival of patients who received the two treatments (51% vs 41%; P = .1) was not signiï¬cant.
A trial conducted by the European Organisation for Research and Treatment of Cancer (EORTC) compared patients not treated with adjuvant chemotherapy with those treated with 3 months of preoperative FOLFOX chemotherapy followed by resection and 3 more months of adjuvant chemotherapy.[9] Patients who received chemotherapy had a longer disease-free survival. In a more recent update, no survival advantage was seen.[45]
A recent trial compared perioperative adjuvant systemic FOLFOX chemotherapy with and without cetuximab in patients with wild-type KRAS.[46] Not only did cetuximab not improve outcomes, use of this agent was associated with an inferior progression-free survival. In a randomized trial of adjuvant therapies after liver resection, Ychou et al[47] found no advantage of FOLFIRI over 5-FU as adjuvant therapy. These trials demonstrate that data from studies of chemotherapy in the palliative setting cannot be directly extrapolated to the adjuvant setting.
Two randomized trials examined the role of regional hepatic arterial infusional (HAI) ï¬oxuridine (FUDR) chemotherapy as adjuvant therapy after hepatectomy for colorectal metastases.[48,49] One trial compared the use of HAI FUDR with no adjuvant therapy,[48] whereas the other compared the use of systemic 5-FU/LV with combined HAI FUDR and systemic 5-FU/LV.[49] Regional chemotherapy resulted in a very high rate of hepatic disease control and improved disease-free survival.[48,49]
These data indicate that adjuvant chemotherapy should be considered for patients with resected hepatic colorectal metastases, particularly those with a high likelihood of recurrence (CRS > 3). In chemotherapy-naive patients, systemic 5-FU/LV is the regimen for which there is the most evidence, and thus is the most reasonable choice. Many oncologists in the United States and Europe, however, are using systemic FOLFOX as the perioperative adjuvant regimen; their decision is supported mainly by data from studies of adjuvant therapy for stage III cancer and by the progression-free survival benefit seen in the EORTC trial.[9] In patients in whom ï¬rst-line chemotherapy has failed, regional FUDR is an option at centers with the expertise to deliver HAI.
Downstaging chemotherapy for converting disease to resectable
Bismuth et al[50] ï¬rst reported that oxaliplatin-based chemotherapy may convert nonresectable disease to resectable. Since then, there have been many reports of the use of FOLFOX or FOLFIRI chemotherapy to convert disease to resectable.[14,50-54] In approximately 15% of patients treated with systemic chemotherapy, the disease converts to “resectable” status. Regional chemotherapy using HAI FUDR is particularly impressive in its ability to convert unresectable disease to resectable. Clavien et al[52] reported a conversion rate of nearly 30% for regional HAI FUDR, whereas Kemeny et al[53] reported a rate of > 50% for a regimen that combined HAI FUDR with systemic FOLFOX (Table 4).
The duration of downstaging chemotherapy before resection is greatly debated. Some investigators think that surgery should occur as soon as the disease is resectable[14]; others believe it should be performed after 4 months, since that seems to be the time of maximum tumor response.[53] It also gives a window before the 9-month time point, which is the median time to progression after the initial response, and it reduces the liver toxicity associated with a long duration of chemotherapy.
The timing can also depend on the need for portal vein embolization (PVE). This technique produces growth of remnant liver prior to resection. The procedure is performed by direct puncture of the portal vein transcutaneously; the vein on the side of the planned future resection is filled with embolic material. Ipsilateral atrophy is accompanied by contralateral hypertrophy. Outcomes of resections performed after preoperative growth of the future remnant liver are therefore improved.[55] It has been shown that chemotherapy does not retard such hypertrophy in a clinically appreciable way and prevents the growth of tumors that may be present on the nonembolized side.[56] In patients who require a lobectomy, we tend to perform PVE early in the course of downstaging chemotherapy. Since the longer the wait, the more the future remnant grows,[57] surgeons sometimes wait a full 6 months while the patient is receiving chemotherapy. The subsequent removal of a very small, atrophied lobe of liver will have negligible impact on the patient’s physiology.
Neoadjuvant chemotherapy: not for all patients
Based on the data for downstaging chemotherapy, clinicians have advocated the use of preoperative, neoadjuvant chemotherapy in initially resectable disease. Following are the main rationales for neoadjuvant therapy:
Some have advocated the use of preoperative chemotherapy as an in vivo chemosensitivity test to determine the response of tumors that have not yet been resected. However, among chemotherapy-naive patients, 60% to 70% respond to bevacizumab and FOLFOX chemotherapy and another 23% may have stable disease.[58] Progression occurs in only 5% of patients. This has been conï¬rmed by a study of perioperative chemotherapy in which progression of disease was noted in only 7% of patients.[9]
Advocates of routine neoadjuvant chemotherapy also contend that the additional waiting period allows distant disease to manifest itself, thereby clarifying when disease is not resectable for cure. In the only multicenter trial involving neoadjuvant chemotherapy, the development of additional tumors that ruled out resection occurred in only 2% of patients.[9] Thus, for chemotherapy-naive patients, the data support neither of these first two reasons.
A period of chemotherapy may also give patients ample time after a recent colectomy to recover before a hepatic resection or can allow for medical optimization in patients with worrisome comorbidities.
On the other hand, immediate resection allows the patient to move along to definitive therapy and avoids the syndrome of liver damage and portal hypertension that has been named chemotherapy-associated steatohepatitis (CASH).[59] There is also increasing evidence that such tissue damage has effects on complications and postoperative recovery after liver resection.[60] Until the data indicate otherwise, neoadjuvant chemotherapy should be used selectively and should not be regarded as the standard of care.
Tumor Ablation: An Effective Minimally Invasive Procedure
Although surgery remains the gold standard for operable liver cancers, there are many circumstances in which surgical resection cannot be offered because of underlying liver disease, comorbidities, or extensive tumor involvement. In these situations, other forms of liver-directed therapies have emerged, including needle-based ablation, transcatheter-based therapies, and radiation therapy.
Focal liver ablation
Needle ablative therapies comprise a wide array of modalities, including percutaneous ethanol ablation, radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, and irreversible electroporation (IRE). All of these minimally invasive ablative techniques can be performed either surgically (open or laparoscopic) or completely percutaneously. Image guidance, either by computerized tomography (CT) or ultrasound, allows optimal treatment planning, visualization and targeting of lesions, and monitoring for completeness of ablation.
Radiofrequency ablation. RFA is the most commonly used focal ablative liver therapy. Initially described by McGahan et al,[61] liver RFA was first performed laparoscopically by Buscarini et al in 1995[62] and percutaneously by Rossi et al in 1995.[63] RFA utilizes an oscillating electrical current (350–500 MHz) delivered by needles (probes) to generate resistive tissue heating.[64] The result of RF energy deposition is frictional heating around the electrodes.
RFA is highly effective in killing liver cancers, especially smaller tumors; however, larger tumors are less effectively treated. In addition, vascular structures greater than 3 mm in diameter have sufficient blood flow to dissipate heat. Tumors located nearby such heat sinks are therefore a challenge to treat.[65] This procedure now is sufficiently widely used that guidelines for its use were published by the American Society of Clinical Oncology.[66]
Microwave ablation. MWA originated in 1986 as a hemostatic technique [67] and was rapidly developed into an ablative option in the treatment of liver cancers.[68] Microwave systems utilize needle-like antennas to generate high-energy electromagnetic (EM) fields with frequencies in the range of 0.9 to 2.6 GHz. These EM fields work directly on the water molecules within tissues, resulting in high energy absorption and tissue heating. Since this heating process does not require direct conduction, MWA does not have the same physical limitations as RFA. Hence, larger tissue volumes can be ablated, even in tissues with poor conductive properties, such as lung, bone, and ablated/charred tissue.[64] Furthermore, since MWA relies on EM fields, multiple applicators can be placed and synchronized, resulting in synergistic overlapping fields and larger ablation areas. Depending on the device, ablation zones can range from very small (using a single antenna) to > 6 cm in the most optimal settings.[69,70] Because MWA operates at higher temperatures than RFA, it is less affected by heat sink effects.
Irreversible electroporation. IRE (Nanoknife) is a relatively newer technology that uses a nonthermal electrical current to elicit its cytotoxic effect. In IRE, a series of rapid microsecond to millisecond pulses of high energy (1,000–2,500 V/cm2) direct current (70–90 pulses) are transmitted along electric fields between parallel electrodes placed 1 to 2 cm apart. This causes irreversible damage in the form of permanent nanopores within the cellular membrane, with subsequent cell death through cellular apoptotic pathways.[71-73] Because IRE is essentially nonthermal,[74,75] it is not as susceptible to heat sink effects involving nearby vessels. Furthermore, since there is very little scarring and no significant thermal injury to extracellular components, it can be used with relative safety on tumors adjacent to heat-sensitive structures, such as vessels, bile ducts, and nerves (Figure 2).[76,77]
The disadvantages of IRE include the small size of the ablation zone, requiring many overlapping ablations for large tumors. The equipment is also expensive. In addition, IRE utilizes high-energy electrical currents and electric fields, which can result in severe muscle twitches, as well as cardiac arrhythmias. Therefore, complete paralysis under general anesthesia and cardiac gating are necessary to safely treat patients.[77]
Clinical data for focal ablation of liver cancer and metastatic liver lesions
RFA has been successfully used to treat both primary liver cancer and liver metastases. It is clear that RFA is most effective in small tumors (< 3 cm) and can produce durable cancer killing. For primary liver malignancies, three prospective randomized, controlled trials have shown the equivalency of RFA with partial hepatectomy for survival in the treatment of small hepatocellular carcinomas.[78-80] These studies demonstrated that RFA was associated with a lower complication rate and more rapid recovery than open hepatectomy. Data for metastatic colorectal cancer are less definitive; nevertheless, RFA is widely used in this setting. RFA or other liver ablation techniques should be considered as effective treatment for small metastatic lesions deep within the liver, where a large parenchymal resection may be necessary for extirpation of tumor. Peripheral lesions should be considered for either laparoscopic/robotic resection or ablation, depending on the expertise of the treatment center. Contraindications to RFA include proximity to adjacent bowel, bile duct, or other organs that can sustain collateral thermal damage. Proximity to a major blood vessel, which may serve as a heat sink and cause treatment failure, is a relative contraindication. This explains the highly variable results seen in the literature for the use of RFA in liver tumors.[81]
MWA is increasingly being used in the treatment of focal liver cancers and is also being used to treat colorectal cancer liver metastases.[82] Because of the ability of MWA to treat both small and larger tumors, and the speed with which such ablations can be accomplished, many centers are beginning to use it as the ablation technique of choice. Early results, however, did not uniformly demonstrate superiority to RFA[83,84] because first-generation microwave ablators, as a result of power considerations, delivered ablation at the 970-MHz frequency. Combined with the early primitive antenna designs, this produced elliptical “sausage”-shaped ablation zones, which often did not cover the tumors well. The current generation of 2.45-GHz MWA units delivers ablations of round areas that are much more predictable. In a recent report of more than 400 ablations for colorectal liver metastases, MWA was shown to be highly effective, durable, and dependent on tumor size.[85] In fact, for tumors of 1 cm or less, ablation achieved durable cancer killing in 98% of treated lesions at 4 years.[85]
Given its unique cytotoxic effects (nonthermal) and purported relative safety with respect to vascular structures and bile ducts, IRE offers many theoretical advantages in the liver over traditional thermal techniques. Thomson et al[77] first reported the safe use of IRE in humans in 2011. Highlighting the safety of IRE, Kingham et al[86] studied the use of IRE in patients (N = 28) with small tumors (median diameter, 1 cm; range, 0.5–5 cm), all of which were close to a major vascular structure (< 1 cm). No life-threatening events were described (supraventricular tachycardia developed in one patient and portal vein thrombosis in another). Of the 65 tumors treated, only 1 tumor demonstrated persistent disease and 3 tumors recurred locally.[86] Eller et al similarly studied local control of liver tumors in perivascular locations.[87] Unlike Kingham’s study, in which the majority of procedures were performed during open operations, Eller’s study primarily looked at image-guided, percutaneous IRE. In addition, primary tumor sizes were larger on average (tumor diameter, 20 ± 5 mm; range, 11–37 mm). The initial technical success rate was 12 of 14 (85%); follow-up for the patients in this trial is still short.
In a retrospective study of the role of IRE in the peribiliary setting, Silk et al[88] looked at 11 patients with 22 liver metastases within 1 cm of a major biliary duct. The median tumor size was 3.0 cm (range, 1.0–4.7 cm). Evaluation of postprocedural laboratory markers and imaging was performed. Liver function test abnormalities developed in three patients, with one proceeding to biliary stent placement.[89] Thus, clinical use of IRE is still at an early stage. It shows promise in allowing ablation of anatomic sites that were previously prohibitively dangerous for thermal ablation.
Combined liver resection and tumor ablation
Focal ablation techniques also allow for the treatment of extensive bilateral liver cancers. Investigators have long suggested combined resection and ablation as a means of treating extensive colorectal liver metastases[90,91] Most of the early adaptors of this combined approach employed it as a last resort. More recently, the philosophy has shifted to using combined resection and ablation as a liver parenchymal preservation method of choice to improve patients’ recovery. Karanicolas et al[92] demonstrated that combining resection and ablation achieves equivalent cancer-related outcomes while decreasing operative time, blood loss, and recovery time in patients with bilateral colorectal liver metastases. An international consortium of four centers recently confirmed these findings (unpublished data, Serge Evrard and Graham Poston) and formulated the acronym CARe (combined ablation and resection) for this next phase in the natural evolution of the local eradication of cancer with the preservation of functional liver.
Radiation Therapy
External beam radiation therapy
Until recently, because of the low tolerance of the liver to radiation, the role of radiation therapy (RT) in the treatment of liver tumors was limited to the palliative setting. Treatments exceeding 30 to 35 Gy to the whole liver by conventional 2 Gy per fraction lead to radiation-induced liver disease (RILD), characterized by fatigue, elevated alkaline phosphatase, tender hepatomegaly, and anicteric ascites within 4 months of treatment.[93] With the innovative technologies of modern RT, the delivery of large doses of highly conformal radiation as a durable treatment to a limited number of metastases has become feasible. Current delivery of RT uses imaging studies for tumor delineation, computerized RT planning, intensity-modulated RT (IMRT), image guidance RT, respiratory motion assessment, and stereotactic targeting technology. Stereotactic body RT (SBRT), which delivers radiation through multiple beams by both coplanar and noncoplanar geometries, has emerged as an effective and safe therapy for patients with oligometastatic liver disease. SBRT is an effective therapy for patients who are not suitable candidates for surgical resection or RFA because of technical or medical reasons.
Breathing-related target motion in the liver can now be assessed by four-dimensional CT scanning and addressed by techniques to immobilize the liver, such as abdominal compression devices, controlled breath holds, gating of the RT beam during certain phases of the respiratory cycle, and tumor tracking via implanted fiducial markers.[94-97] The steep dose gradients from the use of IMRT can spare the surrounding normal tissue.
Blomgren et al[98] from Stockholm, Sweden, published the initial experiences of using SBRT for liver cancers. Twenty-one patients with limited liver metastases were treated with 20 to 45 Gy in 1 to 4 fractions, with a promising 95% local control rate and a mean survival of 17.8 months. No severe liver toxicity was observed. Soon after the study was published, a number of institutions started to use SBRT for liver cancers, and several phase I and II studies have confirmed the safety and efficacy of various RT dose regimens delivered in 1 to 6 fractions. Table 5 summarizes the published results of the prospective trials of SBRT for liver metastases.[99-106] All the trials enrolled patients with adequate hepatic function, treated five or fewer metastases with a maximal tumor size of 6 cm, and used 18 to 60 Gy in 1 to 6 fractions. Although all the trials to date are small in scale and have been confounded by significant heterogeneity in primary sites, number and size of metastases, degrees of prior systemic and/or locoregional treatment, and various RT dose regimens, the reported 56% to 100% 2-year actuarial local control rates are certainly encouraging. Severe toxicity (grade ≥ 3) related to SBRT to the liver was uncommon, and no treatment-related deaths were reported in these trials. Specifically, the risk of RILD was very low as long as normal tissue dose constraints, such as 700 mL of normal liver receiving less than 1,500 cGy in 3 fractions, were followed.[103] It is also recognized that higher SBRT doses are associated with a better local control rate.[100,102,107,108] To achieve a 90% 1-year local control rate, a total prescription dose of 48 to 52 Gy in 3 fractions is recommended.[109] For lesions larger than 3 cm and located close to large vessels or hepatic hilum, a more protracted fractionation of 50 to 60 Gy in 5 fractions is recommended.[100] Currently, the RAS01 trial is testing the efficacy of RFA vs SBRT for patients with liver metastases (http://clinicaltrials.gov/ct2/show/NCT01233544).
Proton beam therapy
The energy of a proton beam is attenuated only slightly as the beam traverses a medium and approaches the end of the beam range, where the energy deposited rises to a high value followed by a rapid falloff to zero. The area of a high dose of energy deposited at the end of the beam is known as the Bragg peak. Beyond the Bragg peak, there is no exit RT dose; therefore, one of the advantages of proton therapy is the marked reduction of radiation damage to surrounding normal tissue. These characteristics allow tumor dose escalation for potentially better local control and at the same time reduce treatment-related toxicity. Since the main concern in the use of conventional SBRT for hepatic metastases is the risk of RILD, the use of proton therapy has theoretical advantages. Indeed, in a dosimetric comparison study of 10 patients with solitary liver metastases, the volume of normal liver that could be spared was significantly higher in the proton treatment plan compared with the conventional RT plan.[110] Of note, published trials using various hypofractionated regimens of proton therapy for hepatocellular carcinoma report local control rates of up to 81% at 5 years.[111-116] So far, no clinical reports of proton therapy for hepatic metastases have been published, although two active prospective clinical trials using proton therapy for liver metastases are ongoing (http://clinicaltrials.gov/ct2/show/NCT01239381; http://clinicaltrials.gov/ct2/show/NCT01697371).
Regional brachytherapy: radioembolization
Transarterial radioembolization (TARE) is also known as selective internal radiation therapy (SIRT). This internal brachytherapy utilizes tiny, radioactive microspheres delivered via a transcatheter route. Unlike true embolic therapies, SIRT does not cause tissue ischemia. Its mechanism of action stems directly from the tumoricidal properties of the attached yttrium-90 radioisotope (90Y), which is a high-energy beta-emitter (mean of 0.93 MeV) with a half-life of 64.1 hours. Tissue penetration of the emitted beta-particles ranges from 2.5 mm to 11 mm, making it relatively safe as a therapeutic radioisotope. Because of the short half-life and minimal tissue penetration of 90Y, special radiation confinement is not necessary after radioembolization treatment, which is now performed as an outpatient procedure at most institutions.
There are two commercially available forms of 90Y microspheres. SIRSpheres (Sirtex Medical, Ltd) couple the 90Y isotope to nondegradable, biocompatible ceramic resin spheres ranging from 20 to 60 microns in diameter, with an average specific activity of ~50 Bq/sphere. In contrast, TheraSpheres (BTG, Nordion) are 90Y-tagged glass microspheres (20–30 µm) with a higher average specific activity of 2,500 Bq/sphere. SIR-Spheres have been approved by the US Food and Drug Administration for the treatment of metastatic colorectal cancer.
In brief, 90Y radioembolization begins with a pretreatment mapping angiogram and a technetium-99 macroaggregated albumin shunting analysis. The purpose of these studies is to identify intrahepatic and extrahepatic shunts that may potentially result in nontarget deposition of the 90Y microspheres, causing radiation gastrointestinal ulcers or perforations or other soft tissue injuries. Radioembolizaton treatments can then be performed using whole liver delivery; sequential lobar treatments, spaced 2 to 3 weeks apart; or segmental delivery (radiosegmentectomy).[117-120]
When used appropriately, radioembolization effectively treats many different types of liver cancer. It clearly can produce a tumor response when delivered as a treatment for metastatic colorectal cancer (Table 6)[121-124] and can even produce sufficient shrinkage of tumors to downstage unresectable cases to resectable.[125] The precise roles of this promising brachytherapy technique in the treatment of patients with resectable and unresectable disease still await the results of trials such as the ongoing SIRFLOX (FOLFOX Plus SIR-Spheres vs FOLFOX Alone in Patients With Liver Metastases From Primary Colorectal Cancer) and FOXFIRE (5-Fluorouracil, Oxaliplatin, and Leucovorin With or Without Radioembolization) studies, which are comparing the use of chemotherapy with and without 90Y in the treatment of nonresectable colorectal metastases.
Treatment of Synchronous Tumors: Staged vs Simultaneous Resections
One-quarter of cases of colorectal cancer present with synchronous liver metastases, and there has been ongoing debate about whether to perform staged or simultaneous resections. The debate centers on issues of safety, cancer outcomes, and quality of life. Studies published as late as the 1990s reported prohibitive mortality of as high as 17% for combining resection of the primary cancer with major hepatectomy.[126] Thus, until recently, surgeons were willing to combine only minor liver resections with resection of the primary colorectal cancer; they were unwilling to combine resection of the liver with an extensive pelvic operation.
Recent years have seen tremendous improvement in the safety of liver resection and hence in that of combined resections. Current data clearly indicate that in well-selected patients such simultaneous resections are safe and allow for reduced time to recovery and to the start of appropriate adjuvant chemotherapy.[127-129] Most recently, simultaneous major hepatectomy and rectal resection has also been shown to be safe.[130]
Remaining contraindications to simultaneous resection include major medical comorbidities, bowel obstruction, bowel perforation, and lack of technical expertise to perform both the liver and colorectal resections.
Algorithms of Care
For patients with colorectal cancer who present with synchronous liver metastases, an algorithm of care is shown in Figure 3. If the patient has synchronous primary and metastatic disease that can be safely removed in the same operation, a combined resection is justiï¬ed.[127,130] If the primary colorectal cancer has already been removed, delay in resection of the liver metastases is medically justified for recovery from the primary resection or if the patient’s comorbidities dictate optimization of medical condition.
For metachronous metastases, preoperative chemotherapy is currently supported by data only if the hepatic lesions are borderline resectable or unresectable based on technical or medical issues (Figure 4). All others should have resection followed by adjuvant chemotherapy except in the clinical trial setting. Those who fail to respond to first-line chemotherapy should be considered for combined regional and systemic chemotherapy.
Financial Disclosure:Dr. Fong is a scientific consultant for Covidien Inc and Johnson and Johnson Inc. The remaining authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Picardo A, Karpoff HM, Ng B, et al. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery. 1998;124:57-64.
2. Ito K, Govindarajan A, Ito H, Fong Y. Surgical treatment of hepatic colorectal metastasis: evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J. 2010;16:103-10.
3. Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346-55.
4. Van CE, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-17.
5. Scheele J. Liver resection for colorectal metastases. World J Surg. 1995;19:59-71.
6. Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254-62.
7. Fong Y, Fortner J, Sun R, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Presented at the American Surgical Association Meeting; 1999.
8. Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829-35.
9. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-16.
10. Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathological risk factors. Surgery. 1994;116:703-11.
11. Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487-99.
12. Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-66.
13. Kato T, Yasui K, Hirai T, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46(suppl 10):S22-31.
14. Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal [liver] metastases. Ann Surg Oncol. 2001;8:347-53.
15. House MG, Ito H, Gonen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744-5.
16. Strasberg SM, Dehdashti F, Siegel BA, et al. Survival of patients evaluated by FDG-PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. Ann Surg. 2001;233:293-9.
17. Figueras J, Torras J, Valls C, et al. Surgical resection of colorectal liver metastases in patients with expanded indications: a single-center experience with 501 patients. Dis Colon Rectum. 2007;50:478-88.
18. Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575-80.
19. Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125-35.
20. Nathan H, de Jong MC, Pulitano C, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755-64, 64-6.
21. Fortner JG, Fong Y. Twenty-five year follow-up for liver resection: the personal series of Dr. Joseph G. Fortner. Ann Surg. 2009;250:908-13.
22. Fong Y, Gonen M, Rubin D, et al. Long-term survival is superior after resection for cancer in high volume centers. Ann Surg. 2005;242:540-7.
23. Fong Y, Blumgart LH, Fortner JG, Brennan MF. Pancreatic or liver resection for malignancy is safe and effective in the elderly. Ann Surg. 1995;222:426-37.
24. Adam R, Frilling A, Elias D, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg. 2010;97:366-76.
25. Weber SM, Jarnagin WR, DeMatteo RP, et al. Survival after resection of multiple hepatic colorectal metastases. Ann Surg Oncol. 2000;7:643-50.
26. Hughes K, Scheele J, Sugarbaker PH. Surgery for colorectal cancer metastatic to the liver. Surg Clin North Am. 1989;69:339-59.
27. Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278-84.
28. Ballantyne GH, Quin J. Surgical treatment of liver metastases in patients with colorectal cancer. Cancer. 1993;71(S12):4252-66.
29. Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13-29.
30. Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:492-505.
31. Stephenson KR, Steinberg SM, Hughes KS, et al. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastasis. Ann Surg. 1988;208:679-87.
32. Mala T, Bohler G, Mathisen O, et al. Hepatic resection for colorectal metastases: can preoperative scoring predict patient outcome? World J Surg. 2002;26:1348-53.
33. Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-8.
34. Schussler-Fiorenza CM, Mahvi DM, Niederhuber J, et al. Clinical risk score correlates with yield of PET scan in patients with colorectal hepatic metastases.
J Gastrointest Surg. 2004;8:150-7.
35. Jarnagin WR, Conlon K, Bodniewicz J, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer. 2001;91:1121-8.
36. Poultsides GA, Bao F, Servais EL, et al. Pathologic response to preoperative chemotherapy in colorectal liver metastases: fibrosis, not necrosis, predicts outcome. Ann Surg Oncol. 2012;19:2797-804.
37. Turcotte S, Katz SC, Shia J, et al. Tumor MHC class I expression improves the prognostic value of T-cell density in resected colorectal liver metastases. Cancer Immunol Res. 2014;2:530-7.
38. Yopp AC, Shia J, Butte JM, et al. CXCR4 expression predicts patient outcome and recurrence patterns after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2012;19(suppl 3):S339-46.
39. Crowe PJ, Yang JL, Berney CR, et al. Genetic markers of survival and liver recurrence after resection of liver metastases from colorectal cancer. World J Surg. 2001;25:996-1001.
40. Ito H, Mo Q, Qin LX, et al. Gene expression profiles accurately predict outcome following liver resection in patients with metastatic colorectal cancer. PloS One. 2013;8:e81680.
41. Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-14.
42. Saltz LB, Clarke S, az-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-9.
43. Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976-82.
44. Park R, Gonen M, Kemeny N, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204:753-61.
45. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-15.
46. Primrose J, Falk S, Finch-Jones M, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601-11.
47. Ychou M, Hohenberger W, Thezenas S, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20:1964-70.
48. Kemeny MM, Adak S, Lipsitz S, et al. Results of the intergroup [Eastern Coorperative Oncology Group (ECOG) and Southwest Oncology Group (SWOG)] prospective randomized study of surgery alone versus continuous hepatic artery infusion of FUDR and continuous systemic infusion of 5FU after hepatic resection for colorectal metastases. Proc Am Soc Clin Oncol. 1999;18:264a.
49. Bozzetti F, Bignami P, Montalto F, et al. Repeated hepatic resection for recurrent metastases from colorectal cancer. Br J Surg. 1992;79:146-8.
50. Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509-20.
51. Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644-57.
52. Clavien PA, Selzner N, Morse M, et al. Downstaging of hepatocellular carcinoma and liver metastases from colorectal cancer by selective intra-arterial chemotherapy. Surgery. 2002;131:433-42.
53. Kemeny NE, Huitzil Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465-71.
54. Alberts SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243-9.
55. Covey AM, Brown KT, Jarnagin WR, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451-5.
56. Fischer C, Melstrom LG, Arnaoutakis D, et al. Chemotherapy after portal vein embolization to protect against tumor growth during liver hypertrophy before hepatectomy. JAMA Surg. 2013;148:1103-8.
57. Correa D, Schwartz L, Jarnagin WR, et al. Kinetics of liver volume changes in the first year after portal vein embolization. Arch Surg. 2010;145:351-4.
58. Tabernero J, Van CE, az-Rubio E, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25:5225-32.
59. Fong Y, Bentrem DJ. CASH (Chemotherapy-Associated Steatohepatitis) costs. Ann Surg. 2006;243:8-9.
60. Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16:137-44.
61. McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267-70.
62. Buscarini L, Rossi S, Fornari F, et al. Laparoscopic ablation of liver adenoma by radiofrequency electrocauthery. Gastrointest Endosc. 1995;41:68-70.
63. Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous radiofrequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J Sci Am. 1995;1:73-81.
64. Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16:192-200.
65. Lu DS, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267-74.
66. Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493-508.
67. Tabuse Y, Tabuse K, Mori K, et al. Percutaneous microwave tissue coagulation in liver biopsy: experimental and clinical studies. Nihon geka hokan Archiv fur japanische Chirurgie. 1986;55:381-92.
68. Yu H, Burke CT. Comparison of percutaneous ablation technologies in the treatment of malignant liver tumors. Semin Intervent Radiol. 2014;31:129-37.
69. Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914-21.
70. Laeseke PF, Lee FT, Jr, van der Weide DW, Brace CL. Multiple-antenna microwave ablation: spatially distributing power improves thermal profiles and reduces invasiveness. J Interv Oncol. 2009;2:65-72.
71. Lee EW, Chen C, Prieto VE, et al. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255:426-33.
72. Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver. 2010;4(suppl 1):S99-104.
73. Lee EW, Wong D, Prikhodko SV, et al. Electron microscopic demonstration and evaluation of irreversible electroporation-induced nanopores on hepatocyte membranes. J Vasc Interv Radiol. 2012;23:107-13.
74. Faroja M, Ahmed M, Appelbaum L, et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology. 2013;266:462-70.
75. Golberg A, Yarmush ML. Nonthermal irreversible electroporation: fundamentals, applications, and challenges. IEEE Trans Biomed Eng. 2013;60:707-14.
76. Lee YJ, Lu DS, Osuagwu F, Lassman C. Irreversible electroporation in porcine liver: short- and long-term effect on the hepatic veins and adjacent tissue by CT with pathological correlation. Invest Radiol. 2012;47:671-5.
77. Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22:611-21.
78. Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-8.
79. Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-12.
80. Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma.
J Hepatol. 2012;57:794-802.
81. Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncology. 2004;5:550-60.
82. Wang J, Liang P, Yu J, et al. Clinical outcome of ultrasound-guided percutaneous microwave ablation on colorectal liver metastases. Oncol Lett. 2014;8:323-6.
83. Ding J, Jing X, Liu J, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013;82:1379-84.
84. Wang ZL, Liang P, Dong BW, et al. Prognostic factors and recurrence of small hepatocellular carcinoma after hepatic resection or microwave ablation: a retrospective study. J Gastrointest Surg. 2008;12:327-37.
85. Leung UKD, D’Angelica M, Kingham P, et al. Longterm survival following microwave ablation for liver malignancies. Br J Surg. In press.
86. Kingham TP, Karkar AM, D’Angelica MI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg. 2012;215:379-87.
87. Eller A, Schmid A, Schmidt J, et al. Local control of perivascular malignant liver lesions using percutaneous irreversible electroporation: initial experiences. Cardiovasc Intervent Radiol. 2014 May 6. [Epub ahead of print]
88. Silk MT, Wimmer T, Lee KS, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25:112-8.
89. Dunki-Jacobs EM, Philips P, Martin Ii RC. Evaluation of thermal injury to liver, pancreas and kidney during irreversible electroporation in an in vivo experimental model. Br J Surg. 2014;101:1113-21.
90. Rivoire M, De CF, Meeus P, et al. Combination of neoadjuvant chemotherapy with cryotherapy and surgical resection for the treatment of unresectable liver metastases from colorectal carcinoma. Cancer. 2002;95:2283-92.
91. Abdalla EK, Vauthey JN. Technique and patient selection, not the needle, determine outcome of percutaneous intervention for hepatocellular carcinoma. Ann Surg Oncol. 2004;11:240-1.
92. Karanicolas PJ, Jarnagin WR, Gonen M, et al. Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA Surgery. 2013;148:597-601.
93. Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237-48.
94. Wunderink W, Mendez Romero A, de Kruijf W, et al. Reduction of respiratory liver tumor motion by abdominal compression in stereotactic body frame, analyzed by tracking fiducial markers implanted in liver. Int J Radiat Oncol Biol Phys. 2008;71:907-15.
95. Dawson LA, Eccles C, Bissonnette JP, Brock KK. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys. 2005;62:1247-52.
96. Wagman R, Yorke E, Ford E, et al. Respiratory gating for liver tumors: use in dose escalation. Int J Radiat Oncol Biol Phys. 2003;55:659-68.
97. Shirato H, Shimizu S, Kitamura K, et al. Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor. Int J Radiat Oncol Biol Phys. 2000;48:435-42.
98. Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncologica. 1995;34:861-70.
99. Scorsetti M, Arcangeli S, Tozzi A, et al. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys. 2013;86:336-42.
100. Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18:1081-7.
101. Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486-93.
102. Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585-91.
103. Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572-8.
104. Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncologica. 2006;45:823-30.
105. Mendez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase i-ii study. Acta Oncologica. 2006;45:831-7.
106. Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164-70.
107. McCammon R, Schefter TE, Gaspar LE, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73:112-8.
108. Wulf J, Guckenberger M, Haedinger U, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncologica. 2006;45:838-47.
109. Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117:4060-9.
110. Petersen JB, Lassen Y, Hansen AT, et al. Normal liver tissue sparing by intensity-modulated proton stereotactic body radiotherapy for solitary liver tumours. Acta Oncologica. 2011;50:823-8.
111. Mizumoto M, Okumura T, Hashimoto T, et al. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys. 2011;81:1039-45.
112. Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053-9.
113. Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115:5499-506.
114. Fukumitsu N, Sugahara S, Nakayama H, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831-6.
115. Kawashima M, Furuse J, Nishio T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23:1839-46.
116. Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127(5 suppl 1):S189-93.
117. Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78-85.
118. Kosmider S, Tan TH, Yip D, et al. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J Vasc Interv Radiol. 2011;22:780-6.
119. van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol. 2009;27:4089-95.
120. Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol). 2007;19:397-417.
121. Gray B, Van HG, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711-20.
122. Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412-25.
123. Stubbs RS, Cannan RJ, Mitchell AW. Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases.
J Gastrointest Surg. 2001;5:294-302.
124. Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135-150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging.
J Vasc Interv Radiol. 2005;16:1641-51.
125. Gulec SA, Pennington K, Hall M, Fong Y. Preoperative Y-90 microsphere selective internal radiation treatment for tumor downsizing and future liver remnant recruitment: a novel approach to improving the safety of major hepatic resections. World J Surg Oncol. 2009;7:6.
126. Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000;231:743-51.
127. Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233-41.
128. Reddy S, Zorzi D, Lum YW, et al. Timing of multimodality therapy for resectable synchronous colorectal liver metastases: a retrospective multi-institutional analysis. Ann Surg Oncol. 2009;16:1809-19.
129. Martin RC, Augenstein V, Reuter NP, et al. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg. 2009;208:842-50.
130. Silberhumer PP, Temple LK, Araujo RLC, et al. Simultaneous resection for rectal cancer with synchronous liver metastasis is a safe procedure. Am J Surg. In press.
131. Kemeny NE, Chou JF, Capanu M, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer. 2014 Aug 25. [Epub ahead of print]
132. Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509-20.
133. Barone C, Nuzzo G, Cassano A, et al. Final analysis of colorectal cancer patients treated with irinotecan and 5-fluorouracil plus folinic acid neoadjuvant chemotherapy for unresectable liver metastases. Br J Cancer. 2007;97:1035-9.
134. Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711-20.
135. Stubbs RS, O’Brien I, Correia MM. Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases: single-centre experience with 100 patients. ANZ J Surg. 2006;76:696-703.
136. Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres--safety, efficacy, and survival. Radiology. 2008;247:507-15.