Visceral Metastases and Prostate Cancer Treatment: ‘Die Hard,’ ‘Tough Neighborhoods,’ or ‘Evil Humors’?
Although the mechanism(s) underlying the relatively poor prognosis of prostate cancer patients with visceral disease have yet to be fully elucidated, these new findings suggest that the microenvironment of bone lesions may be immunologically distinct from those at other sites.
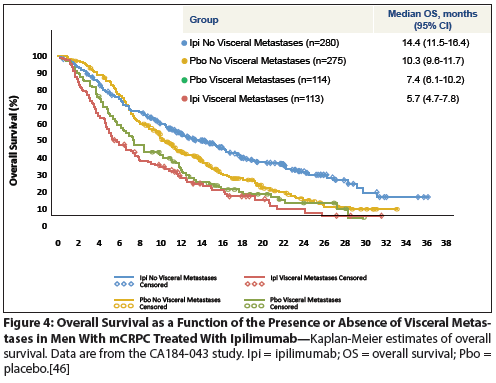
Figure 1: Overall Survival (OS) as a Function of the Site of Metastases in mCRPC
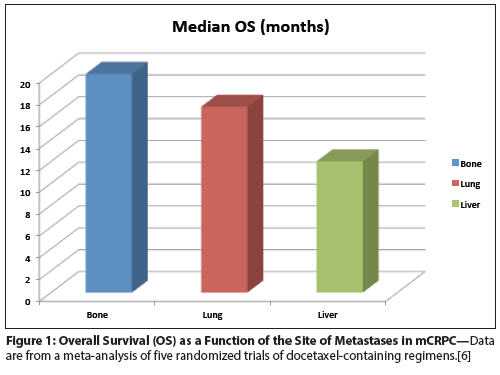
Figure 2: Overall Survival as a Function of the Presence or Absence of Visceral Metastases in Men With mCRPC Treated With Abiraterone Acetate With or Without Prednisone
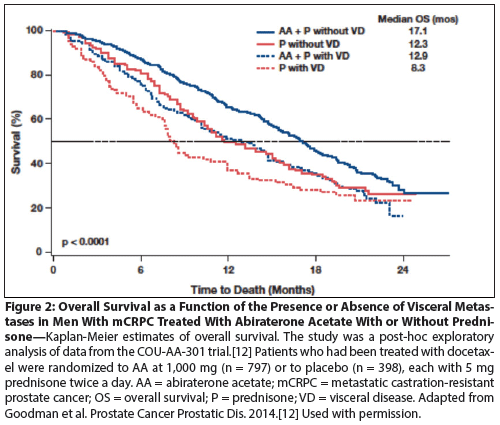
Figure 3: Overall Survival as a Function of the Presence or Absence of Visceral Metastases in Men With mCRPC Treated With Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone
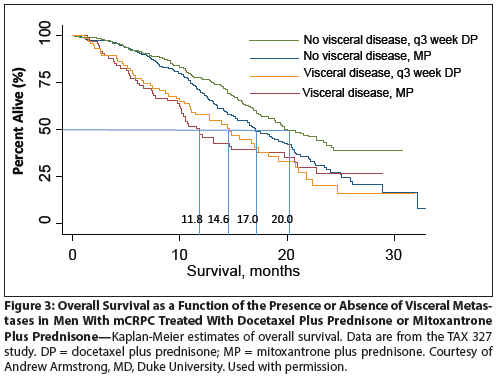
Figure 4: Overall Survival as a Function of the Presence or Absence of Visceral Metastases in Men With mCRPC Treated With Ipilimumab
Men with metastatic castration-resistant prostate cancer have multiple treatment options, and the expanding palate of available therapies renders careful patient selection imperative. Men with visceral (especially hepatic) metastases have a particularly poor prognosis, regardless of the treatment selected. Retrospective analyses of datasets from large phase III randomized trials showed that men with visceral metastases appear to derive clinical benefit from second-generation antiandrogens as well as from docetaxel chemotherapy, but not from immunotherapy. The mechanistic underpinnings of these observations are currently not clear, but could involve factors that are intrinsic to the tumor cell, the tumor microenvironment, and/or systemic factors. Regardless of the underlying mechanism(s), a better understanding of the basic biology of visceral vs bone metastases will be critical in improving prostate cancer treatment in the setting of advanced disease.
Introduction
Metastases to soft-tissue sites other than lymph nodes are common in men with prostate cancer, occurring in approximately 20% of patients participating in first-line studies for metastatic castration-resistant prostate cancer (mCRPC).[1,2] These data are consistent with findings in later stages of disease; a large autopsy series of over 1,500 patients found liver metastases in 25% of patients and lung metastases in 46%.[3] The importance of this observation is highlighted by the fact that the presence of visceral metastases is an independent, negative prognostic factor in men with bone metastases.[4]
Despite the negative implications of visceral metastases for overall survival (OS), retrospective analyses of patients enrolled in clinical trials of either chemotherapy or hormonal therapy showed evidence of clinical benefit in patients with both visceral and bone-only disease. In sharp contrast, recent data from a large randomized phase III trial of ipilimumab, an immune checkpoint–blocking antibody, showed that this may not be the case for immunotherapy.[5] Here, similar retrospective analyses suggested that men with visceral metastases may not derive a clinical benefit from immunotherapy. Because prior studies of immunotherapy for prostate cancer have generally excluded men with visceral metastases, this finding had not been observed previously. Although the mechanism(s) underlying the relatively poor prognosis of men with visceral disease have yet to be fully elucidated, these new findings suggest that the microenvironment of bone lesions may be immunologically distinct from those at other sites.
The Biology of Bone and Visceral Metastases in Prostate Cancer
Despite the high prevalence of visceral metastases in men with mCRPC, and the well-documented association of visceral disease with poor outcomes (Figure 1),[6] few studies have examined cell-intrinsic, tumor microenvironment, or systemic factors that might contribute to the differences between visceral and bone disease in men with mCRPC. The data accumulated thus far suggest that important differences are likely.
In one relevant study, Akfirat et al performed immunohistochemical (IHC) analysis of tissue microarrays to examine the antiapoptotic pathways expressed in visceral vs bone metastases.[7] The results were fascinating, showing that soft-tissue metastases are more likely to express nuclear survivin, whereas bone lesions demonstrated relative overexpression of cytoplasmic survivin, B-cell lymphoma 2 (BCL2), and myeloid cell leukemia 1 (MCL1). Data such as these suggest that drugs aimed at inducing apoptosis in cancer cells may have vastly different efficacies depending on the site of metastasis.
In terms of broader differences in the microenvironment, a small microarray study by Morrissey and colleagues also revealed evidence for physiologically and clinically important differences between bone, liver, and lymph node metastases.[8] Here, soft-tissue lesions derived from liver and lymph nodes were found to express an angiogenic profile different from that of bone metastases, with a significant relative overexpression of the proangiogenic factor angiopoietin-2. These results were verified by IHC studies, which showed that the differences occurred at the protein as well as the message level.
On a systemic level, serum cytokine levels have been associated with prognosis as well as with the presence of liver metastases in several tumor types, including prostate cancer. For example, one study in patients with colorectal cancer showed that systemic levels of transforming growth factor beta 1 (TGF-β1) correlated with the presence of liver metastases post resection.[9] In prostate cancer, a number of studies have examined levels of TGF-β and interleukin-6 (IL-6) as prognostic markers.[10] In particular, the addition of TGF-β and soluble IL-6 receptor levels to a preoperative nomogram significantly improved the ability to predict biochemical progression of prostate cancer.[11] To date, though, no study has comprehensively compared the serum cytokine profiles of prostate cancer patients with vs without visceral metastases.
Visceral Disease: Effects on Response to Hormonal Therapy
Data from a recently published study, COU-AA-301, confirm the findings of multiple previous studies showing that the presence of visceral metastases is associated with decreased OS in the absence of second-generation hormonal treatment. This comprehensive, post-hoc exploratory analysis of men treated in a randomized phase III trial compared abiraterone acetate vs placebo in men with mCRPC who had received docetaxel treatment (Figure 2).[12] In the group that received prednisone alone, median OS was 12.3 months in men without visceral disease vs 8.3 months in men with visceral metastases. However, in terms of treatment benefit, these retrospective analyses showed that abiraterone acetate mediated a similar benefit in men with or without visceral disease: in men without visceral disease, median OS was 17.1 months in the abiraterone-plus-prednisone group, vs 12.3 months for men treated with prednisone alone, corresponding to an increase in median OS of 4.8 months. A strikingly similar OS benefit was noted in men with visceral disease (4.6 months), with survival in both treatment groups less than that in men without visceral metastases (12.9 months for abiraterone acetate vs 8.3 months for placebo). Additional analyses showed that liver metastases were associated with a particularly poor outcome, with an OS of only 6.7 months in the abiraterone-plus-prednisone arm.
Although retrospective analyses such as these must be interpreted with a sufficient degree of caution, they are consistent with data from the AFFIRM trial, a post-docetaxel study of the second-generation antiandrogen enzalutamide.[13] Here, multivariate analyses also showed that the presence of liver metastases was an independent predictor of OS (P < .0002), with treatment showing evidence of benefit regardless of whether or not visceral disease was present. Taken together, these two analyses show that second-line hormonal therapy is a reasonable treatment option for men with mCRPC who have visceral metastases; they also support the notion that the phenotype of prostate cancer metastatic to the liver may be more lethal than that metastatic to the bone.
Visceral Disease: Effects on Response to Chemotherapy
Docetaxel is a standard chemotherapy treatment option for men with mCRPC, as established by two pivotal trials comparing treatment with intravenous docetaxel every 3 weeks vs placebo; treatment in each arm also included prednisone.[1,2] Using data from one of those trials, TAX 327, Armstrong et al performed retrospective analyses aimed at determining the association of baseline factors such as visceral metastases with prognosis.[14] In the docetaxel treatment arm, median OS was 20.8 months for men with bone-only disease vs 13.8 months for men with liver disease. These data are notable in that they are in broad agreement with a prior study,[15] and showed that the presence of liver metastases was an independent prognostic factor for OS by univariate Cox analysis (hazard ratio [HR] = 2.28; 95% confidence interval [CI], 1.73–3.00, P < .001) but not in a multivariate model (HR = 1.66; 95% CI, 1.09–2.54; P = .19). Interestingly, neither visceral metastases nor liver metastases were associated with prostate-specific antigen (PSA) doubling time,[14] suggesting that the association between visceral metastases and OS is not likely to be a simple reflection of the pace of disease progression. Similar to the data on hormonal therapy discussed above, docetaxel treatment appears to convey clinical benefit in men with and without visceral disease, with an OS benefit of approximately 2 to 3 months in both groups (Figure 3). [Andrew Armstrong, MD, unpublished data.] A recently presented meta-analysis of patients treated in several clinical trials of docetaxel chemotherapy strongly confirms the notion that disease site correlates with survival: in an analysis of five docetaxel chemotherapy trials in mCRPC, Halabi et al showed that median OS was approximately 20 months for bone-only disease, 17 months for patients with lung metastases, and only 12 months for men with liver metastases.[6] So, as is the case for second-generation hormonal therapy, men with liver disease likely benefit from chemotherapy treatment, but clearly have a less favorable overall prognosis.
Visceral Disease: Effects on Response to Immunotherapy
Cancer immunotherapy is an emerging treatment option that encompasses multiple modalities, including cancer vaccines and immune checkpoint blockade, as well as the intratumoral or systemic administration of immunostimulatory agents.[16-18]
Cancer vaccines
In prostate cancer, the personalized cancer vaccine sipuleucel-T was approved in 2010 by the US Food and Drug Administration for the treatment of men with asymptomatic or minimally symptomatic mCRPC.[19] This trial excluded men with visceral metastases. Another vaccine regimen for prostate cancer, PROSTVAC-VF,[20] is currently undergoing phase III testing in a large, multinational randomized trial (ClinicalTrials.gov identifier: NCT01322490); that trial also excludes men with visceral disease.
Although the reasoning behind exclusion of these patients from phase III immunotherapy trials has not been made explicit, one logical rationale might involve the generally poor prognosis of such patients; since an antitumor immune response typically takes weeks to months to induce, it seems likely that men with progressive visceral disease may simply not have adequate survival time for a clinically meaningful antitumor immune response to develop.
Immune checkpoint blockade
In addition to cancer vaccines, cancer immunotherapy using immune checkpoint blockade is an attractive treatment modality that capitalizes on the observation that many, if not most, cancer patients have generated endogenous tumor-reactive T cells, but that those cells are held in “check” by a series of cell surface molecules that act like a brake on the immune system.[21] Blocking these negative checkpoints with specific monoclonal antibodies is thus analogous to a driver taking his/her foot off the brakes, allowing an antitumor immune response to proceed. This strategy has been shown to have clinical activity in patients with several tumor types: blockade of the immune checkpoint molecule cytotoxic T-lymphocyte–associated protein 4 (CTLA-4)[22,23] and, more recently, programmed cell death 1 (PD-1)[24,25] have resulted in objective responses.
In the case of mCRPC, immune checkpoint blockade using the CTLA-4 blocking antibody ipilimumab showed reasonable tolerability in a series of phase I and II studies,[26] and a recent provocative case report documented a complete response in one treated patient.[27] Based on these data, CA184-043, a large randomized phase III trial (ClinicalTrials.gov identifier: NCT00861614), was launched in 2009, comparing ipilimumab with placebo in men with mCRPC who had progressed on or after docetaxel chemotherapy. This trial included a small dose (8 Gy) of radiation therapy to one or more sites of metastasis, in an attempt to induce immunogenic antigen release as had been demonstrated in an animal model.[28] Perhaps because the trial focused on patients with late-stage disease, patients with visceral metastases were not excluded from participation. Overall, the trial failed to meet its primary OS endpoint, with an OS of 11.2 months in the ipilimumab group vs 10.0 months in the placebo group (HR = 0.85; 95% CI, 9.5–12.7 for ipilimumab and 8.3–11.0 for placebo; P = .53). There was a statistically significant improvement in progression-free survival (a prespecified secondary endpoint) in the immunotherapy group compared with the placebo group: 4.1 months vs 3.0 months, respectively (HR = 0.70; 95% CI, 3.6–4.3 for ipilimumab and 2.9–3.4 for placebo; P < .0001). Based on stratification factors (alkaline phosphatase level, hemoglobin level, Eastern Cooperative Oncology Group performance status, treatment region) several preplanned post-hoc analyses were conducted. As is the case for any retrospective analysis, the results of such studies must be considered hypothesis-generating rather than definitive. Nevertheless, even in these relatively underpowered analyses, one variable emerged as strongly associated with a lack of benefit from ipilimumab immunotherapy: the presence of visceral metastases. Indeed, as shown in Figure 4, men with visceral metastases appeared to derive no benefit from the treatment, whereas men with no visceral metastases had a median OS of 14.4 months in the treatment group vs 10.3 months in the placebo group. These data are fascinating, suggesting that, unlike hormonal therapy (Figure 2) or chemotherapy (Figure 3), this immunotherapy may not confer benefit in men with mCRPC with visceral metastasis. In terms of future development of CTLA-4 blockade as a therapy for prostate cancer, a second randomized phase III study of ipilimumab (ClinicalTrials.gov identifier: NCT01057810) has completed accrual; this trial enrolled men with earlier-stage (prechemotherapy) disease and specifically excluded men with visceral metastases. Results are expected sometime in 2015.
Potential Mechanisms of Immunotherapy Resistance in Prostate Cancer Patients With Visceral Metastases
Cancer cell intrinsic factors: the ‘die hard’ hypothesis
Although it is unwise to draw conclusions from a retrospective analysis, it is tempting to consider the potential implications of the data from the CA184-043 ipilimumab immunotherapy trial-ie, “If this is true, what does it imply?” Viewed in that light, one possible explanation for the resistance of visceral prostate cancer lesions to immunologic attack could be the differential up-regulation of relevant antiapoptotic pathways in visceral vs nonvisceral metastases. This hypothesis postulates that intrinsically resistant cancer cells preferentially home to or thrive in the liver and/or lungs (the possibility that the liver microenvironment-the “neighborhood”-mediates resistance is discussed below). Indeed, the published data provide some preliminary support for this “die hard” hypothesis,[7] although additional analyses of a larger number of cases with an expanded scope of antiapoptotic pathway members would be required to more fully determine the role of that mechanism. To examine this hypothesis on a functional level, one could also envision employing standard cytotoxic T-cell assays, in which antigen-specific T-cell clones are incubated with patient-derived target tumor cells and lysis of target cells is quantified for the induction of apoptosis. Indeed, such assays can be performed in patients with melanoma, but in prostate cancer the lack of an obvious tumor antigen makes such studies more challenging. Animal studies of prostate cancer are similarly hampered by lack of a model that reliably metastasizes to bone.
A second set of cell-intrinsic factors that could be involved are those relating to the doubling time of visceral prostate cancer cells compared with those in the bone. Because antitumor immune responses take weeks to months to develop, tumors with more rapid doubling times would be expected to be relatively resistant to immunotherapy. Indeed, Ki67 staining, an approximate correlate of cell division, has shown promise as a prognostic biomarker in prostate cancer.[29] Arguing against doubling time as a potential mechanism for resistance to immunotherapy are the data of Armstrong et al, showing overall PSA doubling time was not grossly different between men with bone-only vs visceral disease.[14]
Factors in the tumor microenvironment: the ‘tough neighborhood’ hypothesis
Although not comprehensively investigated thus far, studies published in the peer-reviewed literature[8] and presented at meetings[30] support the notion that the local microenvironment of liver metastases could prove to be vastly different from that of bone lesions. Early studies[8] suggested differences in the angiogenic pathways up-regulated in these disparate locales, with bone metastases preferentially up-regulating angiopoeitin-2. From an immunologic perspective, these data may be more relevant than they first appear, because proangiogenic factors such as vascular endothelial growth factor (VEGF) have been directly demonstrated to down-modulate an antitumor immune response. In patients with melanoma who were treated with ipilimumab, high pretreatment levels of VEGF were associated with poorer OS.[31] Mechanistically, the association between elevated VEGF levels and impaired immunity could be explained by data showing that VEGF inhibits dendritic cell migration, attenuating the initiation of an adaptive immune response. Indeed, blockade of VEGF signaling with monoclonal antibodies improved the efficacy of immunotherapy in an animal model,[32] and a recent study in patients with melanoma showed that the addition of the anti-VEGF antibody bevacizumab to ipilimumab improved T-cell trafficking to tumors.[33]
Other potentially relevant local (“neighborhood”) resistance mechanisms include stromal factors: in pancreatic cancer models, resistance to immunotherapy has been associated with expression of fibroblast activation protein-alpha by cancer-associated fibroblasts.[34] Immunosuppressive cell populations such as regulatory T cells and/or myeloid-derived suppressor cells[35] could also play a role in resistance. Neither of these cell types has been investigated for differential expression in bone vs visceral metastases, although multiple studies showed the expression of regulatory T cells in primary prostate tumors.[36,37]
Systemic cytokines: the ‘evil humors’ hypothesis
Cytokines are small signaling molecules that for the most part are secreted locally but later access the circulation, where they may be detectable in the serum and mediate diverse effects throughout the entire organism. A large number of cytokines have been identified and characterized, and an in-depth discussion of this plethora of molecules is beyond the scope of this review. Among serum cytokines with the potential to mediate systemic immunosuppression in men with prostate cancer and visceral metastases, several are worth considering. Perhaps most extensively investigated in this respect is IL-6, which can be directly secreted from prostate tumor cells as well as macrophages and other cells in the tumor microenvironment. The immunologic role of IL-6 is complex, but one of its major jobs is to steer the adaptive immune response away from a tumoricidal TH1 response and toward an immune response associated with chronic inflammation and tumor growth promotion.[38] Elevated systemic IL-6 levels have been described in a number of studies of prostate cancer patients,[39] and may correlate with the development of cachexia.[40]
Tumor necrosis factor alpha is another systemic proinflammatory cytokine associated with the kind of chronic inflammation that promotes tumor growth[41]; it has also been associated with cachexia in prostate cancer patients.
A third cytokine potentially involved in the association between visceral metastases and poor responsiveness of such patients to immunotherapy is IL-8, a pleotropic cytokine produced by several cell types. Immunologically, IL-8 has a well-documented role in neutrophil recruitment.[42] With regard to this role, it is interesting to note recent data showing that the ratio of neutrophils to lymphocytes has prognostic value in prostate cancer[43] as well as in other malignancies. In addition to a potential role in inflammation-associated tumor promotion, a second mechanism by which IL-8 might promote immunosuppression involves angiogenesis; binding of IL-8 to specific receptors on endothelial cells promotes angiogenesis,[44] which, as described above, is associated with an impaired adaptive immune response.
Finally, TGF-β has a well-documented role in immunosuppression[45] and is systemically elevated in patients with prostate cancer.
Quantifying circulating cytokine levels is reasonably easy, and can be performed using multiplexed arrays with only miniscule amounts of serum. To date, though, no study has specifically examined cytokine levels as a function of the site of metastasis in prostate cancer patients. This is unfortunate, because small molecule and/or antibody inhibition of each of these molecules is achievable and has the potential to work synergistically with other treatment modalities in men with advanced mCRPC.
Clinical Implications, Conclusions, and Clinical Trial Design
From a clinical perspective, the short-term implications of these data are straightforward; men with mCRPC and visceral metastases should not be treated with immunotherapy with sipuleucel-T. If additional immunotherapy agents such as PROSTVAC-VF and/or ipilimumab are granted approval for the treatment of mCRPC, they should not be administered to men with visceral metastases. This conclusion is not challenging to support, because those patients were specifically excluded from the pivotal phase III study of sipuleucel-T,[19] as well as the ongoing studies of the other two immunotherapy agents (ClinicalTrials.gov identifiers: NCT01322490 and NCT01057810). Immunotherapy agents for prostate cancer should be used “on-label.”
In terms of clinical trial design, it would seem prudent for randomized phase II or phase III trials in men with mCRPC to consider including disease site as a stratification factor, so that a preponderance of patients with poor-prognosis visceral metastases is not inadvertently assigned to one arm. Further basic research testing the “die hard” and “tough neighborhood” hypotheses is ongoing and has the potential to identify targetable metabolic and signaling pathways relatively unique to bone vs visceral disease. However, of the three hypotheses presented, the “evil humors” hypothesis is by far the simplest to explore, requiring investigators only to collect pretreatment serum and determine cytokine levels using one of the many reliable and readily available multiplex cytokine analysis platforms. Those studies could possibly identify a predictive serum biomarker to help select patients for treatment, but in a broader sense they have the potential to identify a targetable mechanism underlying the poor performance of immunotherapy in prostate cancer patients with visceral disease.
Financial Disclosure:Dr. Drake has served as a paid consultant to, and has received sponsored research funding from, Bristol-Myers Squibb and Janssen Pharmaceuticals.
References:
1. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer.
N Engl J Med. 2004;351:1513-20.
2. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-12.
3. Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578-83.
4. Fizazi K, Massard C, Smith MR, et al. Baseline covariates impacting overall survival (OS) in a phase III study of men with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2012:30(suppl):abstr 4642.
5. Drake CG, Kwon ED, Fizazi K, et al. Results of subset analyses on overall survival (OS) from study CA184-043: Ipilimumab (Ipi) versus placebo (Pbo) in post-docetaxel metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2014:32(suppl 4):abstr 2.
6. Halabi S, Kelly WK, Zhou H, et al. The site of visceral metastases (mets) to predict overall survival (OS) in castration-resistant prostate cancer (CRPC) patients (pts): A meta-analysis of five phase III trials. J Clin Oncol. 2014:32(suppl 5):abstr 5002.
7. Akfirat C, Zhang X, Ventura A, et al. Tumour cell survival mechanisms in lethal metastatic prostate cancer differ between bone and soft tissue metastases.
J Pathol. 2013;230:291-7.
8. Morrissey C, True LD, Roudier MP, et al. Differential expression of angiogenesis associated genes in prostate cancer bone, liver and lymph node metastases. Clin Exp Metastasis. 2008;25:377-88.
9. Tsushima H, Ito N, Tamura S, et al. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258-62.
10. Steuber T, O’Brien MF, Lilja H. Serum markers for prostate cancer: a rational approach to the literature. Eur Urol. 2008;54:31-40.
11. Kattan MW, Shariat SF, Andrews B, et al. The addition of interleukin-6 soluble receptor and transforming growth factor beta1 improves a preoperative nomogram for predicting biochemical progression in patients with clinically localized prostate cancer. J Clin Oncol. 2003;21:3573-79.
12. Goodman OB, Jr, Flaig TW, Molina A, et al. Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:34-9.
13. Loriot Y, Fizazi K, De Bono JS, et al. Outcomes in patients with liver or lung metastatic castration-resistant prostate cancer (mCRPC) treated with the androgen receptor inhibitor enzalutamide: Results from the phase III AFFIRM trial. J Clin Oncol. 2013;31(suppl):abstr 5065.
14. Armstrong AJ, Garrett-Mayer ES, Yang TC, et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396-403.
15. Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232-7.
16. Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24-37.
17. Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580-93.
18. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480-9.
19. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22.
20. Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001-11.
21. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-64.
22. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
23. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-26.
24. Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N Engl J Med. 2013;369:134-44.
25. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-54.
26. Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813-21.
27. Graff JN, Puri S, Bifulco CB, et al. Sustained complete response to CTLA-4 blockade in a patient with metastatic, castration-resistant prostate cancer. Cancer Immunol Res. 2014;2:399-403.
28. Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728-34.
29. Antonarakis ES, Keizman D, Zhang Z, et al. An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer. 2012;118:6063-71.
30. Hiader M, Coleman I, Zhang X, et al. Does tumor microenvironment affect the response of castration resistant prostate cancer to therapy in bone vs visceral metastases? Proc Am Urological Assoc Annual Meeting. 2014;4(suppl):abstr MP49-15.
31. Yuan J, Zhou J, Dong Z, et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2014; 2:127-32.
32. Manning EA, Ullman JG, Leatherman JM, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951-9.
33. Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632-42.
34. Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827-30.
35. Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer.
J Immunol. 2009;182:4499-506.
36. Miller AM, Lundberg K, Ozenci V, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients.
J Immunol. 2006;177:7398-405.
37. Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254-61.
38. Weaver CT, Harrington LE, Mangan PR, et al. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677-88.
39. Azevedo A, Cunha V, Teixeira AL, Medeiros R. IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World J Clin Oncol. 2011;2:384-96.
40. Pfitzenmaier J, Vessella R, Higano CS, et al. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. 2003;97:1211-6.
41. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-44.
42. Yoshimura T, Matsushima K, Tanaka S, et al. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987;84:9233-7.
43. Nuhn P, Vaghasia AM, Goyal J, et al. Association of pretreatment neutrophil-to-lymphocyte ratio (NLR) and overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with first-line docetaxel. BJU Int. 2013 Oct 29. [Epub ahead of print]
44. Huang S, Mills L, Mian B, et al. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125-34.
45. Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554-67.
46. Drake CG, Kwon ED, Fizazi K, et al. Results of subset analyses on overall survival (OS) from study CA184-043: ipilimumab (Ipi) versus placebo (Pbo) in post-docetaxel metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2014;32(suppl 4):abstr 2.