Lymphoma: Risk and Response After Solid Organ Transplant
Post-transplant lymphoproliferative disorder (PTLD) is a common and serious complication of solid organ transplantation. It is a heterogeneous collection of diagnoses with varied clinical courses and outcomes. The majority of PTLD is Epstein-Barr virus (EBV)-driven as a result of loss of immune control of EBV-positive B lymphocytes. Risk factors for the development of PTLD thus reflect loss or absence of EBV immunity; they include younger age and pre-transplant EBV naivety, as well as the degree and type of immune suppression, type of organ transplantation, and time from transplantation. Identifying patients at risk for PTLD and developing strategies to prevent PTLD is the subject of much research, and the use of antiviral medications and EBV vaccines has yielded intriguing, albeit preliminary, results. As we learn more about the prognostic factors affecting outcome and the pathogenesis of individual diseases, we are better able to tailor therapy to the individual. Further clinical investigation, including randomized controlled trials, will be important in reaching this goal.
Post-transplant lymphoproliferative disorder (PTLD) is a common and serious complication of solid organ transplantation. It is a heterogeneous collection of diagnoses with varied clinical courses and outcomes. The majority of PTLD is Epstein-Barr virus (EBV)-driven as a result of loss of immune control of EBV-positive B lymphocytes. Risk factors for the development of PTLD thus reflect loss or absence of EBV immunity; they include younger age and pre-transplant EBV naivety, as well as the degree and type of immune suppression, type of organ transplantation, and time from transplantation. Identifying patients at risk for PTLD and developing strategies to prevent PTLD is the subject of much research, and the use of antiviral medications and EBV vaccines has yielded intriguing, albeit preliminary, results. As we learn more about the prognostic factors affecting outcome and the pathogenesis of individual diseases, we are better able to tailor therapy to the individual. Further clinical investigation, including randomized controlled trials, will be important in reaching this goal.
TABLE 1

World Health Organization Classification of Post-transplant Lymphoproliferative Disorder (PTLD)FIGURE 1
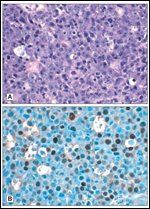
Monomorphic post-transplant lymphoproliferative disorder (PTLD): diffuse large B-cell lymphomaFIGURE 2
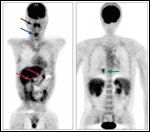
Positron Emission Tomography/Computed Tomography Scan of Multifocal, Extranodal, Monomorphic PTLD in a Patient Post Lung Transplantation for Cystic Fibrosis
Post-transplant lymphoproliferative disorders (PTLDs) are a relatively common and significant complication following solid organ transplantation, occurring in up to 10% of adult patients.[1] They constitute a heterogeneous collection of diagnoses ranging from early lesions, with reactive plasmacytic hyperplasia, to polymorphic PTLD, with polyclonal or monoclonal expansion of atypical lymphoid cells, to monomorphic PTLD, with a frank lymphoma histopathology and phenotype[2] (Table 1, Figure 1). Monomorphic PTLD is most commonly diffuse large B-cell lymphoma, but can also be Burkitt/Burkitt-like lymphoma, myeloma, and less commonly T-cell lymphoma; classical Hodgkin lymphoma-type PTLD is very rare.[2] They differ from non–transplant-related adult lymphomas in that they tend to be extranodal, high grade, and have an aggressive clinical course, with a mortality often exceeding 50% (Figure 2).[1,2] A number of risk factors for the development of PTLD following solid organ transplant have been identified; these have not, however, generally translated into effective prophylactic strategies. With the advent of new lymphoma treatments and with advances in our understanding of adoptive immunity, prognosis following a diagnosis of PTLD is improving. This review will outline PTLD risk factors and strategies for prevention, and PTLD prognosis and strategies to improve treatment outcomes.
FIGURE 3
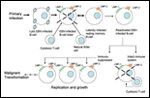
Natural History of Epstein-Barr Virus (EBV) B-cell Infection in Immunocompetent and Immunosuppressed Individuals
Pathogenesis, Epidemiology, and Risk Factors
PTLD following hematopoietic stem cell transplantation is usually a malignancy of donor lymphoid cells, whereas PTLD following solid organ transplantation is traditionally thought to be of recipient origin in the majority of cases, though donor-derived cases have been reported and typically involve the grafted organ.[3,4] In PTLD following both hematopoietic and solid organ transplantation, more than 80% of PTLDs are of B-cell origin.[5] PTLD following solid organ transplantation can occur early, within the first year after transplant, or late, at 1 year or longer following transplantation; the former is much more common, with an incidence of 224 per 100,000 that falls to 54 per 100,000 by the second year.[1] More than 90% of early-onset B-cell PTLD are Epstein-Barr virus (EBV)-positive, whereas over 50% of late-onset B-cell PTLD cases are EBV-negative.[6] Following initial EBV infection, lifelong viral persistence is established within B lymphocytes, which express a combination of 10 viral genes, thereby establishing a latency program.[7] Each latency program is defined by expression of a specific set of viral genes, which vary both in their immunogenicity and their oncogenic transforming potential. LMP-1, for example, is a viral oncogene that induces expression of BCL2 and A20, thus inhibiting apoptosis.[8] In healthy EBV carriers, EBV-specific cytotoxic T lymphocytes (CTLs) kill infected B lymphocytes expressing a more active latency program, thus selecting for infected B lymphocytes in which the viral genome is nearly silenced[7] (Figure 1). Immunosuppression following solid organ transplantation, however, results in loss of this selective pressure, allowing for growth and acquisition of additional transforming mutations such as alterations in c-MYC, BCL-6, p53, and DNA hypermethylation (see Figure 3).[9,10]
EBV serologic status before transplant, as well as the degree and type of immunosuppression following transplant, are therefore important regarding the risk of developing PTLD. Patients who are EBV-naive pre-transplant are more likely to develop PTLD post-transplant as a result of primary EBV infection in an immunosuppressed state, often acquired from the donor organ, with rates reported as high as 24 times those seen in EBV-seropositive patients.[11-13] Similarly, younger age at the time of transplantation has also been correlated with higher rates of PTLD, with pediatric patients having a four- to eight-fold increased risk of PTLD compared with their adult counterparts.[1,12] The higher proportion of EBV-naive patients in the younger cohort has been proposed as an explanation for this observed difference.
Likewise, the degree to which EBV immunity is suppressed or lost in chronic carriers is also a risk factor for developing PTLD. There was an initial observed increase in PTLD following the introduction of cyclosporine into post-transplant immunosuppression regimens, but later studies reported no difference in PTLD rates in patients treated with cyclosporine, compared with azathioprine.[1,14,15] The use of tacrolimus, however, has been associated with a two- to five-fold increase in the rate of PTLD in both adult and pediatric populations, compared with cyclosporine.[1,16,17] The use of muromonab-CD3 (Orthoclone OKT3) and anti-thymocyte globulin (ATG) for antirejection prophylaxis at the time of transplantation or for steroid-refractory acute rejection has also been associated with increased rates of PTLD, with rates three- to four-fold greater than those observed in patients not treated with either of these two drugs.[1] Whether these observed increases in rates of PTLD are due to the specific immunosuppressant rather than a cumulative dose effect is controversial, as patients receiving multiple drugs at higher doses appear to be at the highest risk.[1,18]
TABLE 2

Risk Factors for the Development of PTLD
The proposed effect of cumulative dosing of immunosuppression on rates of PTLD is a potential explanation for the increased risk of PTLD seen during the first year following transplant, when immunosuppression is greatest, and in non–kidney transplant patients who require a higher degree of immune suppression to prevent graft rejection.[1,5,9] Indeed, the incidence of PTLD varies with the type of organ being transplanted; in adult patients this ranges from 1%–3% of kidney and liver transplants, to 1%–6% of heart transplants, 2%–6% of heart-lung transplants, 4%–10% of lung transplants, and up to 20% of small bowel transplants.[9] This is likely to be the result of a combination of the degree of immune suppression and the number of EBV-positive lymphocytes transferred with the transplanted organ, however (Table 2).
Other viral infections have been proposed as potential risk factors in the development of PTLD, including hepatitis C virus (HCV) and cytomegalovirus (CMV).[19-21] Similar to EBV status, CMV-negative patients who receive a CMV-positive organ are 4–6 times more likely to develop PTLD than CMV-positive recipients.[21]
Prevention: Prophylaxis and Early Detection
The identification of certain groups at high risk of developing PTLD following solid organ transplant has resulted in the development and investigation of prophylactic and early detection strategies. Antiviral agents have been studied in both the treatment and prophylaxis settings. In the former, no study has demonstrated a clear benefit, although they may have some efficacy in early or polymorphic disease.[22] Regarding the latter, the use of prophylactic IV ganciclovir for the first 100 days post liver transplant in high-risk EBV-seronegative pediatric patients resulted in no cases of PTLD, compared with a PTLD rate of 10% in low-risk transplant recipients who were given oral acyclovir alone.[23] Additionally, one retrospective study compared PTLD rates between two cohorts: one who received no antiviral prophylaxis and one treated prophylactically with antiviral agents.[24] The investigators noted a reduction in the incidence of PTLD with the use of antiviral prophylaxis, from 4.2% to 0.8%, and none of the 15 high-risk EBV-seronegative patients treated with antiviral therapy developed PTLD. Antiviral therapy did not, however, prevent EBV infection, as 72% of patients treated seroconverted. Antiviral prophylaxis, then, may work by treating EBV in its lytic phase, thus decreasing viral load and subsequent infection of memory B cells and germinal center cells, which are the most prone to undergo oncogenic transformation.[9]
EBV vaccination has also been studied in EBV-seronegative recipients.[25] In a phase I study, 16 pediatric patients who were EBV-naive awaiting kidney transplantation received three injections of the EBV Gp350 vaccine; the vaccine was immunogenic but only 33% of patients developed neutralizing antibodies, and these antibodies were short-lived. Future studies with a prolonged vaccine schedule or improved adjuvants are proposed.
An alternative strategy to limit the morbidity and mortality of PTLD following solid organ transplantation has been to identify patients, following transplant, who are at risk for PTLD, and to detect patients with early-stage PTLD. EBV viral load has been shown to be significantly increased in patients who develop PTLD.[26,27] The use of a rising or increased viral load to alter clinical practice has been investigated following hematopoietic stem cell transplant, with a reduction in immunosuppression and/or preemptive therapy with rituximab or EBV cytotoxic T cells.[28-30] There are reported cases of documented PTLD regression. However, EBV viral load is variably predictive of the development of PTLD in solid organ transplant recipients, and perhaps a better screening strategy is to monitor the relative EBV viral load with respect to EBV-specific T-cell count, which has been shown to predict PTLD in 100% of a small cohort of patients.[31,32] This has not yet translated into studies investigating preemptive changes in clinical management in the solid organ transplant setting.
Prognosis
Survival statistics in PTLD are variable, owing to the heterogeneity of the diagnosis, ranging from early lesions to monomorphic PTLD, and to advances in therapy. Median 1-year and 5-year survival rates are approximately 50%–60% and 30%–40%, respectively, depending on the type of organ transplanted.[1] Reported median overall survival has been 20–30 months.[33-36] Various factors have been investigated to better define an individual’s prognosis and to develop a predictive prognostic index for this diagnosis. In one group of 61 patients with PTLD, performance status (PS) > 2 and EBV nondetection in the tumor were associated with a failure to achieve a complete response (CR) to therapy.[33] Decreased survival was predicted by a PS > 2, more than one site of disease, PTLD of T-cell origin, monoclonality, nondetection of EBV, and treatment with chemotherapy. Patients could be separated into low-, intermediate-, and high-risk groups based on PS and number of involved sites. Median survival had not been reached in the low-risk group, was 34 months in the intermediate-risk group, and was 1 month in the high-risk group. This index was more predictive of outcome than the International Prognostic Index (IPI) in this study. In their cohort of 107 adult solid organ transplant recipients, the Mayo Clinic reported an association between poor PS, one or more sites of extranodal disease, high IPI, advanced stage, and elevated lactate dehydrogenase (LDH), and decreased overall survival.[34] They propose a multivariate model for survival based on poor performance status, monomorphic disease, and graft organ involvement. In a group of 60 solid organ transplant recipients with PTLD treated with rituximab monotherapy, age at diagnosis, performance status, LDH, and time from transplantation to PTLD were associated with overall survival, whereas LDH and time from transplantation to PTLD were associated with progression-free survival.[37] While IPI was not predictive in this cohort, a PTLD-specific prognostic index of age > 60 years, ECOG PS > 2, and elevated LDH effectively separated patients into low- (zero risk factors), intermediate- (one risk factor), and high-risk (two to three risk factors) groups, with a 2-year overall survival rate of 88%, 50%, and 0%, respectively. Although the standardized therapy of a large number of patients makes this latest prognostic index appealing, no defined or accepted prognostic index has been developed to date.
TABLE 3
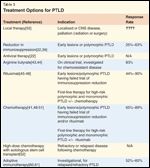
Treatment Options for PTLD
Specific Therapies and Outcomes
There are no established treatment recommendations for PTLD given the heterogeneity of the diagnosis, from pathology to prognosis, and the general lack of prospective, randomized studies in the field. As we have learned more about the varied natural history of the different diseases as well as their risk factors and prognostic factors, therapy can now be better tailored to the individual patient. For instance, a stepwise approach to therapy is often indicated for patients with either early lesions or polymorphic disease, starting with a reduction in immunosuppression with or without the addition of antiviral therapy, to single-agent rituximab, to chemotherapy if indicated. For patients with monomorphic disease, the initial reduction in immunosuppression is typically accompanied by the addition of rituximab with or without chemotherapy, depending on the aggressiveness and histopathology of the disease. Without a defined prognostic index for this disease, however, much of the decision regarding how to treat this disease rests in the hands of the individual physician (Table 3). While guidelines have been published, these have not been based on randomized controlled trials.[38]
Reduction in Immunosuppression
Given the role that immunosuppression and loss of an EBV-specific CTL response play in the development of PTLD, one of the earliest treatment strategies was to decrease the level of immunosuppression following diagnosis. This has been shown to be effective in inducing disease remission in up to 25%–63% of adult patients, depending on the type of PTLD.[22,39] In the only prospective study available, however, a reduction in immunosuppression coupled with high-dose acyclovir for the initial treatment of PTLD resulted in only one partial response amongst 16 patients.[40] All of these patients had monomorphic disease, and the majority had diffuse large B cell lymphoma following heart transplant. A reduction in immunosuppression alone may be sufficient for early lesions and polyclonal PTLDs, whereas monoclonal PTLDs invariably require further antitumor therapy.[41] One study found that an elevated LDH, organ dysfunction, and multiorgan involvement by PTLD were all associated with a lack of response to immunosuppression reduction.[39] A reduction in immunosuppression remains the first step in therapy for nearly all cases of PTLD; this typically involves 25%–50% reductions in cyclosporine and tacrolimus and discontinuation of azathioprine and mycophenolate mofetil.[42]
Antiviral Therapy
Like a reduction in immunosuppression, the use of antiviral therapy in the treatment of PTLD may be limited to patients with early lesions or polymorphic PTLD.[22] One reason antiviral therapy may not be effective in the treatment of PTLD is that the target of these agents, viral DNA polymerase, requires the activity of viral thymidine kinase, which is not expressed in latently infected lymphoma cells. The addition of arginine butyrate, an activator of latently infected lymphoma cells via induction of EBV thymidine kinase, to ganciclovir in patients with disease insensitive to either radiation and/or chemotherapy resulted in a CR in five of six patients and a partial response (PR) in the final patient.[43] A larger phase I/II study of arginine butyrate and ganciclovir in 15 patients with refractory EBV-positive lymphoma included 6 patients with PTLD.[44] There was a response rate of 67% observed in the entire patient population, with 83% of refractory PTLD patients achieving a CR or PR. These results are intriguing, although in the United States arginine butyrate for the treatment of B-cell malignancies is available only in the context of a clinical trial.
Antibody Therapy
The efficacy of the anti-CD20 monoclonal antibody rituximab in B-cell lymphomas, and its relatively benign toxicity profile, made it an attractive agent to investigate in B-cell PTLD. In the first series of 17 patients treated with rituximab, all but one had controlled disease or a response to therapy, and 53% had a CR.[45] The mean duration of response was 35 months, and 3-year overall survival was 56%. Approximately half of these patients had stage IV disease and almost 60% had diffuse large cell pathology. Prognostic factors of response to therapy were EBV positivity and decreased time from transplantation. Two subsequent prospective phase II trials reported response rates of 79% and 44%, respectively.[46,47] The first study consisted of 38 patients, 80% of whom had monomorphic disease, and all of whom were treated with rituximab upfront; progression-free and overall survival at a follow-up of 27.5 months were 42% and 47%, respectively. It is interesting that the three patients who did not respond to rituximab went on to receive chemotherapy, and all had an eventual CR. The 43 patients in the second study received rituximab only after a trial of tapering immunosuppression failed. Sixty-five percent of these patients had monomorphic disease. OS at 1 year for the entire study population was 67%, and median survival was 15 months. In this study, only a normal LDH correlated with response to therapy, and only the number of sites involved correlated with survival. In both trials, therapy with rituximab was safe and well tolerated. A retrospective analysis of 80 patients with PTLD following solid organ transplantation found that treatment with rituximab improved 3-year progression-free and overall survival to 70% and 73% from 21% and 33%, respectively. In addition, CNS involvement, bone marrow involvement, and hypoalbuminemia were predictive of worse outcomes in this cohort.[48] Most recently, interim results of a multicenter prospective phase II trial of 104 patients with PTLD treated with rituximab followed by either consolidation rituximab for complete response or CHOP (cyclophosphamide, adriamycin, vincristine, prednisone) chemotherapy for partial response, stable disease, or progressive disease, were reported.[49] There was a response rate of 54% following rituximab alone (32% CR rate), and response rates were 90% and 89% with consolidation rituximab and CHOP chemotherapy, respectively. One year progression-free survival was similarly encouraging in the consolidation rituximab and CHOP chemotherapy groups, at 90% and 86%, respectively, and both treatment strategies seemed similarly well-tolerated. Interestingly, the majority of these patients had advanced stage, late-onset monomorphic disease with an elevated LDH, and only about half had EBV-positive tumors, suggesting a more high-risk phenotype. Rituximab is now commonly used monotherapy in the setting of a failed trial of immunosuppression tapering, or as adjunctive therapy to chemotherapy for patients with more aggressive or refractory disease.
Chemotherapy
Systemic chemotherapy is typically used for disease that either does not respond to a reduction in immunosuppression and/or rituximab monotherapy, or that is advanced or of an aggressive phenotype at diagnosis. The majority of reported studies were published prior to the routine use of rituximab.[41,50-52] CHOP is the most commonly employed regimen. CHOP has been associated with a response rate of 65%, overall survival of 13.5 months, and progression-free survival of 42 months, in a cohort of patients who failed to respond to a reduction in immunosuppression.[50] Of note, 40% of patients who achieved a CR in this study following CHOP therapy were disease-free at 10 years. The largest series of patients with refractory PTLD treated with chemotherapy reported 5-year survival rates of 24%, 25%, 32%, and 5% for CHOP, PROMACE CytaBOM (cyclophosphamide, doxorubicin, etoposide, prednisone, cytarabine, bleomycin, vincristine, methotrexate, leukovorin), other multidrug regimens, and single-agent chemotherapy, respectively.[52] The main limitation to such aggressive chemotherapy, however, is a high incidence of neutropenia and associated sepsis and death in these already immunosuppressed and heavily pretreated individuals.
Hematopoeitic Stem Cell Transplantation
There are reported cases of successful salvage therapy for relapsed PTLD with high-dose chemotherapy and autologous stem cell transplant.[53] At 30 months follow-up, these patients remain in a CR with preserved graft function. This is a small case series, however, and the majority of patients with relapsed PTLD are not candidates for such aggressive therapy, owing to poor performance status, comorbid conditions, and/or organ dysfunction.
Surgery and Radiation Therapy
As in management of other types of lymphoma, radiation therapy is typically reserved for isolated lesions, either for curative intent in localized disease or for palliation therapy in generalized disease.[54,55] It is also useful for patients with CNS involvement who are typically not candidates for high-dose methotrexate, because of impaired renal function or other comorbidities. In one series of patients, local radiotherapy to the brain induced approximately 82% of all CRs observed, and one-third of such patients in a CR remained alive at 5 years.[56] Involvement of the CNS by PTLD, however, is considered a poor prognostic factor, with overall survival typically on the order of 10%–20%.[56] Surgical therapy, on the other hand, has been used for localized PTLD, sometimes involving removal of a non–life-sustaining graft.[38,52] If these patients can tolerate a reduction in immunosuppression and/or antitumor therapy, however, this may be unnecessary.
Adoptive Immunotherapy
The use of donor-derived EBV-specific CTL lines to treat PTLD following hematopoietic stem cell transplant has proven very promising.[57] This approach is feasible in this setting since it is usually a donor-derived malignancy arising from the new donor-derived, and thus HLA-identical, immune system. For solid organ transplant recipients who were EBV-seropositive prior to transplant, a recipient EBV-specific CTL cell line can be established,
but this is often rate-limiting as it can take up to 14 weeks and is not possible for previously EBV-seronegative patients.[58,59] Additionally, when used in a series of 12 patients either at high risk for PTLD or with frank PTLD, there was only one CR and one PR observed in the PTLD population.[60] Instead, the use of partially HLA-matched EBV-specific CTL lines has been investigated, with three out of seven complete responses seen in patients with relapsed or refractory polyclonal disease.[61] A larger phase II multicenter clinical trial of 33 patients with relapsed or refractory PTLD treated with best HLA-matched EBV-specific CTL lines resulted in a response rate of 52%.[62] Further study regarding the fate of these cells once transfused, however, does not show expansion into memory-type cells and instead these cells exist in circulation only transiently.[63] Long-term follow-up is needed, therefore, to assess for relapse rate and survival.
Reference Guide
Therapeutic Agents
Mentioned in This Article
Acyclovir
Anti-thymocyte globulin (ATG)
Arginine butyrate
Azathioprine (Azasan, Imuran)
CHOP (cyclophosphamide,
adriamycin, vincristine,
prednisone)
Cyclosporine
Ganciclovir
Methotrexate
Muromonab-CD3 (Orthoclone OKT3)
Mycophenolate mofetil
PROMACE CytaBOM
(cyclophosphamide, doxorubicin,
etoposide, prednisone,
cytarabine, bleomycin,
vincristine, methotrexate,
leukovorin)
Rituximab (Rituxan)
Tacrolimus
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
Other therapies
As not every solid organ transplant patient develops PTLD, other factors must contribute to the development of this disease, including the local cytokine environment. IL-6 antibodies have been investigated in a small study of 12 patients, with a response rate of 42%.[64,65] A larger trial is necessary to potentially influence clinical practice. Interferon-α has also been investigated as an adjunct to immunosuppression reduction, with 57% of patients entering a CR.[66] It is poorly tolerated, however, and increases the risk of graft rejection, and is therefore not typically used in the treatment of PTLD.
Lastly, rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR) increasingly being used following both solid organ and bone marrow transplantation, has shown in vitro activity against EBV-transformed B-lymphocyte lines and in vivo activity against the development of tumors in SCID mice injected with EBV-transformed B-cell lines.[67] It has been used in combination with rituximab with success, but whether this is better than rituximab alone is debatable, and there have been reports of patients developing PTLD while on rapamycin for immunosup-
pression.[68,69] An investigation of the OPTN/UNOS database for kidney transplants from 2000–2004 found that there was a statistically significant association between the use of rapamycin and the development of PTLD.[70]
Summary
Post-transplant lymphoproliferative disorder remains one of the most serious and life-threatening complications of solid organ transplantation. It is a heterogenous group of diseases with varied clinical presentations, natural histories, and prognoses. The majority are B-cell neoplasms that are EBV-positive, resulting from either de novo EBV infection in an immunocompromised host, or reactivation of latent EBV infection due to loss of immune control. As such, risk factors for the development of PTLD include EBV seronegative status pre-transplant, age at transplant, type and degree of immunosuppression, type of organ transplanted, and time from transplantation; other concurrent viral infections like HCV and CMV may contribute as well. As we learn more about the risk factors associated with the development of PTLD, the prognoses of the individual diseases, and the pathogenesis of these disorders, we are better able to identify high-risk individuals who might benefit from prophylactic or early detection strategies, and to stratify patients to individualized therapies based on their prognosis. More rigorous investigation, including randomized clinical trials, will be pivotal in achieving these aims.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transpl. 2003;4:222-230.
2. Swerdlow SH, Webber SA, Chadburn A, Ferry JA. Post-transplant lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours and Haematopoietic and Lymphoid Tissue. Lyon: IARC Press, 2008:343-349.
3. Weissman DJ, Ferry JA, Harris NL, et al. Posttransplantation lymphoproliferative disorders in solid organ recipients are predominantly aggressive tumors of host origin. Am J Clin Path. 1995;103:748-752.
4. Petit B, Le Meur Y, Jaccard A, et al. Influence of host-recipient origin on clinical aspects of posttransplantation lymphoproliferative disorders in kidney transplantation. Transplantation. 2002;265-271.
5. Morrison VA, Dunn DL, Manivel JC, et al. Clinical characteristics of post-transplant lymphoproliferative disorders. Am J Med. 1994;97:14-24.
6. Preikasaitis JK, Cockfield SM. Epstein-Barr virus infection and lymphoproliferative disease after hematopoietic stem cell or solid organ transplantation. In: Bowden RA, Ljungman P, Paya CV, et al, eds. Transplant Infections. 2nd Edition. Lippincott Williams & Wilkins, 2003:326-49.
7. Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481-492.
8. Kuppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3:801-812.
9. Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol/Hematol. 2005;56:155-167.
10. Capello D, Rossi D, Gaidano G. Post-transplant lymphoproliferative disorders: molecular basis of disease histogenesis and pathogenesis. Hematol Oncol. 2005;23:61-67.
11. Walker RC, Marshall WF, Strickler JG, et al. Pretransplantation assessment of the risk of lymphoproliferative disorders. Blood. 2003;102:2775-3785.
12. Dror Y, Greenberg M, Taylor G, et al. Lymphoproliferative disorders after organ transplantation in children. Transplantation. 1999;67:990-998.
13. Shapiro R, Nalesnik M, McCauley J, et al. Pottransplant lymphoproliferative disorders in adult and pediatric renal transplant patients receiving tacrolimus-based immunosuppression. Transplantation. 1999;68:1851-1854.
14. Penn I. Cancers following cyclosporine therapy. Transpl Proc. 1987;19:2211-2213.
15. Swinnen LJ, Costanzo-Nordin MR, Fisher SG, et al. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac transplant recipients. N Engl J Med. 1990;323:1723-1728.
16. Cao S, Cox KL, Berquist W, et al. Long-term outcomes in pediatric liver recipients: comparison between cyclosporine A and tacrolimus. Pediatr Transplant. 1999;3:22-26.
17. Caillard S, Dharnidharka V, Agodoa L, et al. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in the era of modern immunosuppression. Transplantation. 2005;80:1233-1243.
18. Melosky B, Karim M, Chui A, et al. Lymphoproliferative disorders after renal transplantation in patients receiving triple or quadruple immunosuppression. J Am Soc Nephrol. 1992;2:S290-S294.
19. Hezode C, Duvoux C, Germanidis G, et al. Role of hepatitis C virus in lymphproliferative disorders after liver transplantation. Hepatology. 1999;30:775-778.
20. Manez R, Breinig MC, Linden P, et al. Post-transplant lymphoproliferative disease in primary Epstein-Barr virus infection after liver transplantation: the role of cytomegalovirus disease. J Infect Dis. 1997;176:1462-1467.
21. Walker RC, Marshall WF, Strickler JG, et al. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995;20:1346-1353.
22. Starzl TE, Porter KA, Iwatsuki S, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporine-steroid therapy. The Lancet. 1984;1:583-587.
23. McDiarmid SV, Jordan S, Kim GS, et al. Prevention and preemptive therapy of posttransplant lymphoproliferative disease in pediatric liver recipients. Transplantation. 1998;66:1604-1611.
24. Malouf MA, Chhajed PN, Hopkins P, et al. Antiviral prophylaxis reduces the incidence of lymphoproliferative disease in lung transplant recipients. J Heart Lung Transplant. 2002;21:547-554.
25. Rees L, Tizard EJ, Morgan AJ, et al. A phase I trial of Epstein-Barr Virus Gp350 vaccine for children with chronic kidney disease awaiting transplantation. Transplantation. 2009;88:1025-1029.
26. Riddler SA, Breinig MC, McKnight JL. Increased levels of circulating Epstein-Barr virus (EBV)-infected lymphocytes and decreased EBV nuclear antigen antibody responses are associated with the development of posttransplant lymphoproliferative disease in solid-organ transplant recipients. Blood. 1994;84:972-984.
27. Kenagy DN, Schlesinger Y, Weck K, et al. Epstein-Barr virus DNA in peripheral blood leukocytes of patients with posttransplant lymphoproliferative disease. Transplantation. 1995;60:547-554.
28. Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 1995;345:9-13.
29. Gustafsson A, Levitsky V, Zou JZ, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95:807-814.
30. Styczynski J, Einsele H, Gil L, et al. Outcome of treatment of Epstein-Barr virus-related posttransplant lymphoproliferative disorder in hematopoietic stem cell recipients: a compreshensive review of reported cases. Transpl Infect Dis. 2009;11:383-392.
31. Hopwood PA, Brooks L, Parratt R, et al. Persistent Epstein-Barr virus infection: unrestricted latent and lytic viral gene expression in healthy immunosuppressed transplant recipients. Transplantation. 2002;74:194-202.
32. Smets F, Latinne D, Bazin H, et al. Ratio between Epstein-Barr viral load and anti-Epstein-Barr virus specific T-cell response as a predictive marker of posttransplant lymphoproliferative disease. Transplantation. 2002;73:1603-1610.
33. Leblond V, Dhedin N, Bruneel M-FM, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19:772-778.
34. Ghobrial IM, Habermann TM, Maurer MJ, et al. Prognostic analysis for survival in adult solid organ transplant recipients with post-transplant lymphoproliferative disorders. J Clin Oncol. 2005;23:7574-7582.
35. Ghobrial IM, Habermann TM, Ristow KM, et al. Prognostic factors in patients with post-transplant lymphoproliferative disorders (PTLD) in the rituximab era. Leuk Lymph. 2005;46:191-196.
36. Oton AB, Wang H, Leleu X, et al. Clinical and pathological prognostic markers for survival in adult patients with post-transplant lymphoproliferative disorders in solid transplant. Leuk Lymph. 2008;49:1738-1744
37. Choquet S, Oertel S, LeBlond V, et al. Rituximab in the management of post-tranpslantation lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol. 2007;86:599-607.
38. Parker A, Bowles K, Bradley JA, et al. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients – BCSH and BTS Guidelines. Br J Haematol. 2010;149:693-705.
39. Tsai DE, Hardy CL, Tomaszewski JE, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71:1076-1088.
40. Swinnen LJ, LeBlanc M, Grogan TM, et al. Prospective study of sequential reduction in immunosuppression, interferon alpha-2B, and chemotherapy for posttransplant lymphoproliferative disorder. Transplantation. 2008;86:215-222.
41. Muti G, Cantoni S, Oreste P, et al. Post-transplant lymphoproliferative disorders: improved outcome after clinico-pathologically tailored treatment. Haematologica. 2002;87:67-77.
42. Paya CV, Fung JJ, Nalesnik MA, et al. Epstein-Barr virus-induced post-transplant lymphoproliferative disorders. ASTS/ASTP EBV-PTLD Task Force and The Mayo Clinic Organized International consensus Development Meeting. Transplantation. 1999;68:1517-1525.
43. Mentzer SJ, Perrine SP, Faller DV. Epstein-Barr virus post-transplant lymphoproliferative disease and virus-specific therapy: pharmacological re-activation of viral target genese with arginine butyrate. Transpl Infect Dis. 2001;3:177-185.
44. Perrine SP, Hermine O, Small T, et al. A phase I/II trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood. 2007;109:2571-2578.
45. Oertel SHK, Verschuuren E, Reinke P, et al. Effect of Anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD). Am J Transpl. 2005;5:2901-2906.
46. Gonzalez-Barca E, Domngo-Domenech E, Capote JE, et al. Prospective phase II trial of extended treatement with rituximab in patients with B-cell post-transplant lymphoproliferative disease. Haematoligica. 2007;92:1489-1494.
47. Choquet S, Leblond V, Herbrecht R, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective, multicenter phase 2 study. Blood. 2006:3053-3057.
48. Evens AM, David KA, Helenowski I, et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28:1038-46.
49. Trappe R, Choquet S, Oertel SHK, et al. Sequential treatment with rituximab and CHOP chemotherapy in B-cell PTLD – Moving forward to a first standard of care: results from a prospective international multicenter trial. ASH abstract 2009:100.
50. Choquet S, Trappe R, Leblond V, et al. CHOP-21 for the treatment of post-transplant lymphoproliferative disorders (PTLD) following solid organ transplantation. Haematologica. 2007;92:273-274.
51. Swinnen LJ. Durable remission after aggressive chemotherapy for post-cardiac transplant lymphoproliferation. Leuk Lymphoma. 1997;28:89-101.
52. Buell JF, Gross TG, Hanaway MJ, et al. Chemotherpay for posttransplant lymphoproliferative disorder: the Israel Penn International Transplant Tumor Registry experience. Transplant Proc. 2005;37:956-957.
53. Komrokji RS, Oliva JL, Zand M, et al. Mini-BEAM and autologous hematopoietic stem-cell transplant for treatment of post-transplant lymphoprolierative disorders. Am J Hematol. 2005;79:211-215.
54. Koffman BH, Kennedy AS, Heyman M, et al. Use of radiation in posttransplant lymphoproliferative disorder (PTLD) after liver transplantation. Int J Cancer. 2000;90:104-109.
55. Kang SK, Kirkpatrick JP, Halperin EC. Low-dose radiation for posttransplant lymphoproliferative disorder. Am J Clin Oncol. 2003;26:210-214.
56. Penn I, Porat G. Central nervous system lymphoma in organ allograft recipients. Transplantation. 1995;59:240-244.
57. Liu Z, Savoldo B, Huls H, et al. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the prevention and treatment of EBV-associated post-transplant lymphomas. Recent Results Cancer Res. 2002;159:123-133.
58. Feng S, Buell JF, Chari RS, et al. Tumors and transplantation: the 2003 third annual ASTS state-of-the-art winter symposium. Am J Transplant. 2003;3:1481-1487.
59. Comoli P, Maccario R, Locatelli F, et al. Treatment of EBV-related post-renal transplant lymphoproliferative disease with a tailored regimen including EBV-specific T cells. Am J Transplant. 2005;5:1415-1422.
60. Savoldo B, Goss JA, Hammer MM, et al. Treatment of solide organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs). Blood. 2006;108:2942-2949.
61. Haque T, Wilkie GM, Taylor C, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360:436-42.
62. Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123-1131.
63. Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative diosreders. Annu Rev Med. 2004.
64. Tosato G, Jones K, Breinig MK, et al. Interleukin-6 production in posttransplant lymphoproliferative disease. J Clin Invest. 1993;91:2806-2814.
65. Haddad E, Paczensny S, Leblond V, et al. Treatment of B-lymphoproliferative disorder with a monoclonal anti-interleukin-6 antibody in 12 patients: a multicenter phase 1-2 clinical trial. Blood. 2001;97:1590-1597.
66. Davis CL, Wood BL, Sabath DE, et al. Interferon-alpha treatment of posttransplant lymphoproliferative disorder in recipients of solid organ transplants. Transplantation. 1998;66:1770-1779.
67. Nepomuceno RR, Balatoni CE, Natkunam Y, et al. Rapamycin inhibits the interleukin 10 signal transduction pathway and growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472-4480.
68. Garcia VD, Bonamigo Filho JL, Neumann J, et al. Rituximab in association with rapamycin for post-transplant lymphoproliferative disease treatment. Transpl Int. 2003;16:202-206.
69. Hymes LC, Warshaw BL. Sirolimus in pediatric patients: results in the first 6 months post-renal transplant. Pediatr Transplant. 2005;9:520-522.
70. Kirk AD, Cherikh WS, Ring M. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant. 2007;7:2619-2625.