Pediatric Anaplastic Large Cell Lymphoma Presenting as Generalized Lymphadenopathy
Here we present the case of a 3-year-old girl with generalized lymphadenopathy and fever, in whom the cause of the symptoms was initially thought to be infectious. Ultimately, however, anaplastic large cell lymphoma (ALCL) was diagnosed. Using this case as a backdrop, we discuss the wide range of systemic illnesses that the differential diagnosis of generalized lymphadenopathy encompasses.
Because a finding of generalized lymphadenopathy can be associated with such a wide range of diseases and conditions, determining its cause can sometimes be challenging. Infectious causes are the most common; however, it is important also to consider other entities in the workup. Here we present the case of a 3-year-old girl with generalized lymphadenopathy and fever, in whom the cause of the symptoms was initially thought to be infectious. Ultimately, however, anaplastic large cell lymphoma (ALCL) was diagnosed. Using this case as a backdrop, we discuss the wide range of systemic illnesses that the differential diagnosis of generalized lymphadenopathy encompasses-including infectious, autoimmune, and oncological disorders. We discuss the different findings typically seen in the various entities that figure prominently in the differential, and we outline investigations that can help narrow it. Finally, we present an overview of ALCL, one of the more rare pediatric malignancies.
Generalized lymphadenopathy is a common finding and has an extensive differential diagnosis. Because generalized lymphadenopathy often has an infectious origin, other less common causes, especially neoplastic causes, can easily be missed.
Here we report the case of a toddler who presented with inguinal lymphadenopathy and fever and in whom supraclavicular, cervical, and axillary lymphadenopathy developed several days later. Initially, bacterial lymphadenitis was diagnosed; however, an infectious disease workup revealed no evidence of either a bacterial or viral infection. Oncological causes were then investigated, and ultimately, anaplastic large cell lymphoma was diagnosed.
Case Report
A 3-year-old girl presented with a 10-day history of “swelling around her vagina” and difficulty walking for 3 days. Her mother had first noted this nontender swelling over the patient’s mons pubis region while bathing her, and on the day of presentation, two tender “bumps” had developed in the inguinal area. Over the past 2 weeks, she had had tactile fevers, night sweats, and fatigue. She also appeared to have lost weight. There was no known tuberculosis (TB) or animal exposure and no travel history.
History
The child had been well until 4 months earlier. At that time-and at another hospital-she received intravenous gammaglobulin for presumed Kawasaki disease (she had right cervical lymphadenopathy, an erythematous rash, and chapped lips). Her symptoms appeared to resolve with this therapy.
Three weeks before her latest visit, the patient had been admitted to yet another hospital for evaluation of refusal to walk; she underwent workup for a septic hip. Ultrasonography, MRI, and two joint aspirations showed no conclusive evidence of a joint infection. However, she was found to be anemic. She was treated with a 2-week regimen of clindamycin, and her symptoms improved.
Initial Evaluation
Her physical exam on admission was significant for warm, tender, firm inguinal masses bilaterally that measured approximately 6 cm on each side. She had no other masses, lymphadenopathy, or hepatosplenomegaly. Breath sounds at the left lung base were decreased. She could bear weight but was reluctant to move her hips because of pain.
The admission complete blood cell (CBC) count revealed the following values: hemoglobin, 9.2 g/dL; hematocrit, 30%; mean corpuscular volume, 61.9 fL; mean corpuscular hemoblobin, 19 pg; reticulocyte count, 1.3%; white blood cell count, 15,500/µL with 55% neutrophils, 43% lymphocytes, and 2% monocytes; and platelet count, 34,100/µL.
Hemoglobin electrophoresis revealed a normal AA pattern with normal quantitative hemoglobin A2 level (2.8%) and an elevated level of hemoglobin F (2.2%). Levels of electrolytes, liver enzymes, lactate dehydrogenase, and uric acid were within normal limits. Serum iron level and iron binding capacity were both low; serum ferritin level was normal. C-Reactive protein (CRP) level was elevated at 5.7 mg/dL; erythrocyte sedimentation rate (ESR) was 82 mm/hr.
Orthopedic consultation and ultrasound evaluation of her hips did not suggest a septic joint.
FIGURE
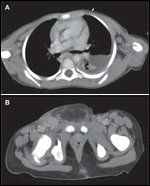
Anaplastic large cell lymphoma.
In this patient, computed tomography (CT) of the chest
(A)
demonstrates a left pleural effusion and bilateral axillary lymphadenopathy measuring up to 7.6 cm. CT of the pelvis
(B)
demonstrates inguinal lymphadenopathy measuring up to 2.4 × 1.4 × 2 cm at the right groin.
Hospital Course
Initially, acute bacterial lymphadenitis was diagnosed. Intravenous antibiotics were started, and the patient’s inguinal adenopathy improved minimally. An infectious disease workup was initiated, including placement of a purified protein derivative (PPD) test, measurement of Epstein-Barr virus (EBV) and coccidioidomycosis titers, a rapid HIV antibody test, Bartonella serologies, and blood polymerase chain reaction (PCR) testing for cytomegalovirus (CMV).
On the third hospital day, the patient was noted to have new supraclavicular, cervical, and axillary lymphadenopathy. Concern for an oncological process prompted computed tomography (CT) imaging of the head, neck, chest, abdomen, and pelvis; the scan revealed a large left pleural effusion and left cervical, left supraclavicular, bilateral axillary, diffuse abdominal, and bilateral inguinal lymphadenopathy (Figure). On gallium scanning, the abnormal regions on the CT scan were gallium avid. Bilateral bone marrow aspirates and biopsies were negative for malignancy. Examination of the pleural fluid did not demonstrate any malignant cells. Her cerebrospinal fluid (CSF) was acellular, and cytology evaluation did not reveal any malignant cells. Results of a subsequent biopsy of the right inguinal lymph node were diagnostic of anaplastic large cell lymphoma (ALCL), lymphohistiocytic variant. This patient’s ALCL was anaplastic lymphoma kinase (ALK)–positive on immunohistochemical staining. However, neither cytogenetics nor fluorescence in situ hybridization demonstrated any translocations involving chromosome 2 (the location of the ALK gene) that might have accounted for the expression of the ALK protein.
Discussion
Differential Diagnosis of Generalized Lymphadenopathy
This patient’s presentation of lymphadenopathy was classified as “generalized,” given its localization to more than two noncontiguous areas, including the inguinal, intra-abdominal, axillary, and cervical regions.[1,2] The differential diagnosis of generalized lymphadenopathy is broad and is generally focused on systemic illnesses, including infectious, neoplastic, and autoimmune disorders.[3]
The patient’s elevated ESR, CRP level, and fever could be indicative of an infectious process, which is the most likely cause of generalized lymphadenopathy. Infectious mononucleosis usually presents with the triad of fever, pharyngitis, and lymphadenopathy.[4] Patients also commonly present with fatigue, malaise, rash, and splenomegaly. The nodal involvement pattern that is characteristic of infectious mononucleosis includes symmetric posterior cervical nodes and anterior cervical nodes; the axillary and inguinal lymph nodes can also be involved. The CBC count typically reveals an elevated lymphocyte count with atypical lymphocytosis, and transaminase elevations are frequently seen on the chemistry profile. The disease is caused by EBV. Because the heterophil antibody (Monospot) test has only a 69% sensitivity during the first week of an infection, EBV serology is a more accurate method for diagnosing an acute infection.[5] This patient had IgG to viral capsid antigen and to nuclear antigen, findings that are usually indicative of a past EBV infection. However, she also had an equivocal level of IgM to viral capsid antigen; this finding can be indicative of a recent infection or of reactivation of a prior infection. On occasion, CMV infection, toxoplasmosis, and hepatitis B infection can cause a mononucleosis-like syndrome. Results of blood PCR for CMV were negative in this patient.
HIV also frequently causes lymphadenopathy. During the second week of acute symptomatic HIV infection, nontender adenopathy involves primarily the axillary, cervical, and occipital nodes. This patient’s symptoms prompted us to perform a rapid HIV antibody test; however, the results were negative.[6]
Miliary TB, the widespread hematogenous dissemination of Mycobacterium tuberculosis, is a serious infectious cause of generalized lymphadenopathy.[7] The presentation can mimic that of malignancy and include weakness, fatigue, weight loss, night sweats, lymphadenopathy, and hepatosplenomegaly. In addition, miliary disease is difficult to detect in the young and is nearly universally fatal if untreated; a high level of suspicion for potential cases is thus warranted. Results of this patient’s PPD test were negative; however, less than 50% of patients with miliary TB have a positive PPD test. Further testing was thus performed, including cultures of blood, pleural fluid, CSF, bone marrow aspirate, and a lymph node biopsy specimen; all results were negative for TB.
Coccidioidomycosis is another potential infectious cause of systemic lymphadenopathy.[8] The presentation can be diverse but has five primary manifestations: acute pneumonia, chronic progressive pneumonia, pulmonary nodules and cavities, extrapulmonary nonmeningeal disease, and meningitis. This patient did reside in an area in which coccidioidomycosis was endemic. However, her serum titers were ultimately negative for this entity.
Although infections are a common cause of generalized lymphadenopathy, one must also consider autoimmune diseases as possible causes. Disorders such as systemic lupus erythematosus, juvenile idiopathic arthritis, and dermatomyositis often cause generalized lymphadenopathy. However, this patient did not have any of the other stigmata of the rheumatologic illnesses, such as arthritis, rash, or mucocutaneous ulcers.[9]
A third major category of disease that must be considered in patients with generalized lymphadenopathy is malignancy. Acute leukemia, the most common malignancy in childhood, accounts for approximately 30% of all childhood cancers.[1,10] However, a bone marrow aspiration was performed in this patient, and the results were negative for leukemia.
Lymphomas are the third most common pediatric malignancy, accounting for 15% of all childhood cancers, and they often present with lymphadenopathy.[11] Non-Hodgkin lymphoma (NHL) comprises approximately 60% of these diagnoses, while Hodgkin disease accounts for the rest. The distribution of NHL in childhood is 40% Burkitt lymphoma (B-cell in origin), 20% diffuse large B-cell lymphoma, 30% lymphoblastic lymphoma (predominantly T-cell in origin), and 10% ALCL.[12]
ALCL: Overview
The World Health Organization’s classification system further subdivides ALCL into two entities. When confined to the skin, the tumor is referred to as primary cutaneous ALCL, and when it presents with systemic disease, it is called systemic ALCL.[12] In the pediatric population, primary cutaneous disease is very rare. Systemic ALCL has a bimodal distribution, with one peak in childhood/young adulthood and another in late adulthood.
The vast majority of ALCL cells are positive for CD30, which is also strongly expressed on Reed-Sternberg cells, seen in Hodgkin disease; in addition, 90% to 95% of ALCL cells express ALK, usually-although not in this case-in association with an ALK-related translocation. ALK is a receptor tyrosine kinase that is normally expressed in neural cells but not in lymphoid cells. The translocation seen in ALCL is typically between the ALK gene on chromosome 2p23 and the nucleophosmin (NPM) gene on 5q35. ALK is important in the development of the brain and other specific neurons in the nervous system. In patients who test positive for ALK expression, the aberrant expression of ALK in lymphoid cells is thought to be necessary but not sufficient to cause malignant transformation. Other changes in the ALK-expressing lymphoid cells lead to full malignant transformation. ALK positivity in patients with ALCL has been associated with better prognosis.[13]
The presenting signs and symptoms of ALCL can be deceptive. As was the case here, 75% of patients present with systemic symptoms, including high fever; night sweats and weight loss are other systemic symptoms that may be seen. Peripheral adenopathy is more common in ALCL than in other forms of pediatric NHL. Extranodal disease is also prevalent, with the potential for skin, bone, soft tissue, lung, mediastinal, liver, and rarely, gut and central nervous system involvement.[14] The presentation of systemic symptoms and tender peripheral adenopathy often leads to an erroneous assumption of an infectious process, and it may be only after the patient fails to improve with antibiotic therapy that evaluation for malignancy is initiated. This was the case here. The patient’s hip pain may actually have been referred pain from her enlarging and tender inguinal lymph nodes. Once the correct diagnosis was made and she started chemotherapy, her pain abated as the adenopathy resolved.
Treatment
The patient was treated with the APO (vincristine, doxorubicin, prednisone) protocol that is used to treat patients with large B-cell lymphomas and peripheral T-cell lymphomas.[15] Because of her young age and concern for cardiotoxicity, she was also given the cardioprotective agent dexrazoxane before each dose of doxorubicin administered during the induction and maintenance phases of therapy.[16] During the maintenance phase, she also received 6-mercaptopurine, and when a cumulative dose of doxorubicin of 300 mg/M2 was attained, intravenous methotrexate was substituted for doxorubicin. For central nervous system prophylaxis, she received intrathecal methotrexate.
Follow-up
The patient promptly went into remission, completed all therapy within 12 months, and continues to be well 21 months after completion of therapy. Her serial physical examinations and follow-up CT and gallium scans show no evidence of recurrence. Cardiac function was normal before start of therapy, remained normal throughout therapy, and continues to be normal after therapy.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Malogolowkin M, Quinn JJ, Siegel SE, Steuber CP. Clinical assessment and differential diagnosis of the child with suspected cancer. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 145-59.
2. Kliegman RM, Behrman RE, Jenson HB, Stanton BF. Nelson textbook of pediatrics. Philadelphia,:Saunders Elsevier; 2007. p. 2093.
3. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313.
4. Friedmann AM. Evaluation and management of lymphadenopathy in children. Pediatr Rev. 2008;20:53-59.
5. Vincent MT, Celestin N, Hussain AN. Pharyngitis. Am Fam Physician. 2004;69:1468.
6. Gaines H, Von Sydow M, Pehrson PO, et al. Clinical picture of primary HIV infection presenting as a glandular fever-like illness. BMJ. 1988;297:1363.
7. Golden MP, Vikram HR. Extrapulmonary tuberculosis. Am Fam Physician. 2005;72:1767.
8. Parish JM, Blair JE. Coccidioidal infection. Mayo Clin Proc. 2008;83:343-48.
9. Calguneri M, Ozturk MA, Osbalkan Z, et al. Frequency of lymphadenopathy in rheumatoid arthritis and systemic lupus erythematosus. J Int Med Res. 2003;31:345-349.
10. Ries LAG, Eisner MP, Kosary CL, et al, editors. SEER Cancer Statistics Review, 1973-1999. Bethesda, Md: National Cancer Institute; 2002. p. 467. Available at http://seer.cancer/gov/csr/1973_1999/. Accessed July 21, 2010.
11. Shukla NN, Trippett TM. Non-Hodgkin lymphoma in children and adolescents. Curr Oncol Rep. 2006;8:387-94.
12. Cairo MS, Raetz E, Lim MS, et al. Childhood and adolescent non-Hodgkin lymphoma: new insights in biology and critical challenges for the future. Pediatr Blood Cancer. 2005;45:753-69.
13. Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281-84.
14. Stein H, Foss HD, Durkop H, et al. CD30+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681-94.
15. Laver JH, Mahmoud H, Pick TE, et al. Results of a randomized phase III trial in children and adolescents with advanced stage diffuse large cell non Hodgkin’s lymphoma: a Pediatric Oncology Group study. Leuk Lymphoma. 2002;43:105-9.
16. Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145-53.