Multimodality Therapy for Esophageal Cancer
Recent developments in the epidemiology, staging, and treatment of esophageal and gastroesophageal junction cancers have led to significant changes in the way these malignancies are managed. Although a relationship between gastroesophageal reflux disease and esophageal cancer has been demonstrated, antireflux surgery has been shown to have no preventive effect with regard to the development of esophageal adenocarcinoma. The newly modified staging system of the World Esophageal Cancer Consortium has helped define the optimal number of lymph nodes to dissect during an esophagectomy. Incorporating modern techniques, such as esophageal ultrasound, fine needle aspiration, and positron emission tomography, can improve the prognostic value of staging. Use of higher-volume centers and higher-volume surgeons for the performance of procedures in upper gastrointestinal cancers is associated with better outcomes. Neoadjuvant chemoradiation using a wide variety of chemotherapy regimens appears to have become the new standard of care for stage II and III esophageal cancer.
Recent developments in the epidemiology, staging, and treatment of esophageal and gastroesophageal junction cancers have led to significant changes in the way these malignancies are managed. Although a relationship between gastroesophageal reflux disease and esophageal cancer has been demonstrated, antireflux surgery has been shown to have no preventive effect with regard to the development of esophageal adenocarcinoma. The newly modified staging system of the World Esophageal Cancer Consortium has helped define the optimal number of lymph nodes to dissect during an esophagectomy. Incorporating modern techniques, such as esophageal ultrasound, fine needle aspiration, and positron emission tomography, can improve the prognostic value of staging. Use of higher-volume centers and higher-volume surgeons for the performance of procedures in upper gastrointestinal cancers is associated with better outcomes. Neoadjuvant chemoradiation using a wide variety of chemotherapy regimens appears to have become the new standard of care for stage II and III esophageal cancer.
There have been practice-changing developments in the treatment of esophageal and gastroesophageal junction (GEJ) cancers over the past several years. New directions have been reported in the epidemiology, staging, and treatment of these cancers. This review will update the reader on the latest in all aspects of the management of these malignancies, including staging, surgery, and multimodality therapy. Particular attention will be paid to recently published and unpublished data.
Epidemiology
Tobacco smoking is a well-established risk factor for the development of squamous cell carcinomas and adenocarcinomas of the esophagus. A study by Duan and colleagues evaluated the intensity of passive smoking exposure by counting the number of smokers who smoked in a participant’s presence for at least one year and measuring the duration of each exposure. The tobacco exposure of 1,716 participants was compared to that in twice as many controls. The authors found no evidence that in persons who have never actively smoked, exposure to passive smoke during their childhood years or during their adult years influences their risk of esophageal adenocarcinoma.[1]
Human papillomavirus has now been implicated in esophageal cancer in reports from both Europe and China, where there is an increasing association, primarily with squamous cell carcinomas.[2] A recent study from the Swedish authors who first described the relationships between gastroesophageal reflux disease, obesity, and esophageal cancer made the observation that antireflux surgery had no preventive effect on the development of esophageal adenocarcinoma. In a population-based cohort study comparing 14,102 people who had had antireflux surgery (representing 120,514 person-years at risk) with the population at large, the overall risk of esophageal adenocarcinoma (n=39) was 12 times higher in those who had had the surgery; no decrease in risk was found with time after antireflux surgery (P=.86).[3]
Staging and Prognosis
Peritoneal Washings
The impact of positive peritoneal washings on gastric cancer outcomes was evaluated retrospectively in patients with gastric cancer who underwent laparoscopy with peritoneal washings. Of these patients, 198 had peritoneal or visceral metastases at the time of laparoscopy and were designated as having M1 disease. The remaining 93 patients had M0cyt+ disease, defined as cytology indicative of metastatic tumor but with no visible visceral or peritoneal metastases. Patients with M0cyt+ tumors had a significantly longer disease-specific survival (DSS) than did those with M1cyt+ disease (P<.0001). Patients with M0cyt+ disease who received subsequent chemotherapy resulting in a conversion to negative cytology at next laparoscopy had better DSS.[4]
Sentinel Node Mapping
Sentinel node mapping is a strategy whose value has been validated in the surgical management of patients with melanoma and breast cancer; it has been shown to reduce complications resulting from unnecessary lymph node dissections. A similar strategy, recently described for lung cancer,[5] has been proposed for gastric and esophageal cancers. A recent study enrolled 433 patients with early gastric cancer whose disease was limited to T1 or T2,N0,M0 tumors; all tumors were less than 4 cm in size, and patients had received no prior therapies. A dual-tracer method that employed radioactive colloid and blue dye was used to detect involved nodes. The rate of detection of involved sentinel lymph nodes was 387/397 (97.5%). The mean number of sentinel nodes per case was 5.6. Use of sentinel node status for the detection of metastases has been reported to have a 93% sensitivity.[6] Further development of the sentinel node concept is ongoing, and this approach may represent a feasible and clinically reasonable option for reducing the extent of resection in the treatment of very early stage esophageal and gastric cancers.
Revised Staging System
In several papers published since 2008, the World Esophageal Cancer Consortium (WECC) has presented a newly modified staging system for esophageal cancer (Table). The revised system includes significant changes in definitions for lymph node (LN) involvement as well as stage groupings for this disease. For the first time, the staging system has been based on a large cohort of patients who underwent surgical resection using modern staging techniques and for whom good clinical annotations were available. The essential changes to the system include the addition of tumor grade and of N (node)1 and N2 categories based on the number of LNs involved (fewer than 5, or 5 or more).[7]
TABLE
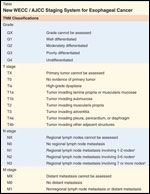
New WECC/AJCC Staging System for Esophageal Cancer
Using WECC data, the relationship between survival and extent of lymphadenectomy (LND) have been described, and the optimum LND defined. A total of 4,627 patients with esophageal cancer who were treated with esophagectomy alone were identified. The risk-adjusted 5-year survival was averaged for each number of lymph nodes resected, and the optimum number of nodes that should be resected to maximize 5-year survival was determined. In moderately and poorly differentiated pN0M0 cancers and in all node-positive (pN+) cancers, 5-year survival improved with increasing extent of LND. In pN0M0 cancers, no optimum LND was defined for pTis; optimum LND was 10 to 12 nodes for pT1, 15 to 22 nodes for pT2, and 31 to 42 nodes for pT3/T4 (depending on histopathologic cell type). In pN+M0 cancers with 1 to 6 positive nodes, the optimum LND was 10 nodes for pT1, 15 nodes for pT2, and 29 to 50 nodes for pT3/T4. Greater extent of LND was associated with increased survival for all patients with esophageal cancer except at the extremes of disease severity (TisN0M0 cancers, and 7 or more regional lymph nodes positive for cancer) and in well-differentiated pN0M0 cancer. Maximum 5-year survival is modulated by T classification: resecting 10 nodes for pT1, 20 nodes for pT2, and 30 or more nodes for pT3/T4 is recommended.[8]
The proposed WECC esophageal system is superior to the current American Joint Committee on Cancer (AJCC) gastric and esophageal staging systems for resected GEJ tumors regardless of neoadjuvant status. The WECC system incorporates GEJ tumors into the esophageal staging system and has the potential for harmonization with the gastric staging system. Unfortunately, there are still challenges involved in using this system as a pretreatment clinical tool in patients with esophageal cancer.[9-11]
Use of Modern Techniques for Staging
In addition, recent reports have emphasized the importance of using modern techniques such as esophageal ultrasound (EUS) and EUS fine needle aspirations (FNA) in pretreatment clinical staging. The use of restaging after neoadjuvant treatment has also advanced and has resulted in the identification of subgroups of patients who may or may not benefit from surgery and/or chemoradiation.[12] The postchemoradiation percentage decrease from baseline in the standardized uptake value (SUV) of positron emission tomography (PET) was shown to correlate with overall survival (OS) and pathologic response. Baseline and postchemoradiation PET/computed tomography (CT) studies were performed in 151 consecutive patients with gastroesophageal adenocarcinoma who had been treated with chemoradiation and surgery. A decrease in SUV of more than 52% was associated with a longer OS (P=.023) and a lower risk of death (P<.01). Pathologic response (50% or less residual cancer) was also associated with longer OS (P=.003). In the multivariate model, the percentage SUV decrease was the only prognostic indicator of OS (P=.01). The percentage SUV decrease was not associated with pathologic complete response.
Tumor Length
The significance of esophageal tumor length has been evaluated in 133 patients with pT1 adenocarcinoma of the esophagus who were undergoing esophageal resection. Patients with early-stage pT1 tumors longer than 3 cm demonstrated decreased long-term survival and higher risk of LN involvement (P<.001). Multivariable analysis showed that esophageal tumor length greater than 3 cm is an independent risk factor for survival in patients with pT1 early-stage esophageal cancer
(P<.001) even when controlled for submucosal involvement, LN involvement, and lymphatic/vascular invasion status. In combination with submucosal involvement, esophageal tumor length greater than 3 cm identified a high-risk population of pT1 esophageal adenocarcinoma.[13]
How Esophageal Cancers Are Staged at Our Institution
Our current approach includes the use of routine CT and PET/CT prior to any other testing to rule out distant metastases and to help localize LN spread. Following this, EUS with possible FNA guided to the suspicious LN lesion is employed. If the suspicious node or nodes are positive, this will allow careful planning of the radiation fields as well as indicate the use of neoadjuvant chemoradiation as opposed to chemotherapy alone. Following the imaging studies, a staging thoracosocpy and/or laparoscopy can be done to confirm early-stage disease if LNs are negative-or to insure that positive LNs are included in the radiation field.
Surgery
Recent articles on esophagectomy have stressed the importance of high-volume, high-quality surgeons and centers. The ability of the promotion of hospital adoption of evidence-based, procedure-specific process measures to improve surgical outcomes was analyzed (eg, routine beta-blockade was studied in 2,780 esophagectomies, 6,267 gastrectomies, and 10,210 lobectomies). Leapfrog standards had no effect on adjusted mortality rates for these high-risk operations, including esophagectomy and gastrectomy (P>.05). The improvements in outcomes that have resulted from the adoption of evidence-based process measures are procedure-specific and do not necessarily reflect overall hospital quality.[14] The benefit of centralization of specialized surgical treatment of upper gastrointestinal cancers in high-volume centers was analyzed in patients in Scotland undergoing esophagectomy and gastrectomy. Hospital mortality rates declined during the study period: in esophagectomy, from 11.7% to 7.9%; in gastrectomy, from 11.2% to 7.2%. For all resections except gastrectomy, mortality decreased as the quartile of hospital-year volume increased. For esophagectomy, the odds ratios of death were lower (P=.009) in hospital years within the highest-volume quartiles than they were in years within the lowest-volume quartile.[15]
An association between higher surgeon volume and improved patient outcomes was reported when procedures with strong surgeon volume–outcome associations in the literature, including esophagectomy and gastrectomy, were studied. There was a significant increase in the proportion of procedures performed by high-volume surgeons over time, with the most dramatic increases seen for gastrectomy (54%).[16]
The use of minimally invasive approaches to esophageal cancer resection, although an attractive alternative to traditional open surgery, raises concerns regarding feasibility, safety, cost, and outcomes; widespread acceptance of these procedures has thus been limited. In a recent study, inpatient mortality and overall surgical morbidity were identical for both the transthoracic open (TTO) cohort and the minimally invasive (MIE) cohort: mortality, 3% versus 2%; morbidity, 50% versus 48%. Pulmonary-related complications were higher in the TTO group (23% versus 8%; P=.05). The incidence of gastric conduit–related complications was similar in the two cohorts (13% versus 18%; P=.52). Survival at 1 and 2 years was 86% and 58%, respectively, in the TTO group and 94% and 74%, respectively, in the MIE group.[17]
A recent report reviewed the records of 750 patients who underwent transhiatal esophagectomy (THE), including 690 (92%) who had malignancies (5.2% located in the upper esophagus, 7.4% in the middle esophagus, 19% in the lower esophagus, and 68.4% at the cardioesophageal junction). The overall in-hospital mortality rate was 2.93% (22 patients). There were no intraoperative deaths. Major complications included atelectasis or pneumonia (4.8%), pleural effusion (22.7%), myocardial infarction (0.5%), recurrent laryngeal nerve paralysis (1.33%), and tracheal laceration (0.4%). The anastamotic leak rate decreased gradually over time from 29.4% to 11.1% in the last 6 years. The average intraoperative blood loss was 315 mL, and 82% of the patients did not receive any blood transfusion. Late functional results were good or excellent in 93% of cases. The average length of hospital stay was 11 days, and the average intensive care unit stay was 2.3 days. The actuarial 5-year survival rate after THE for carcinoma was 21%.[18]
Use of Preoperative and Postoperative Therapy
Following the Walsh trial[19] and the subsequent Cancer and Leukemia Group B (CALGB) 9781 randomized trial[20], chemoradiation is apparently being used as the standard of care for locally advanced esophageal cancer-at least in the United States. Further evidence of the value of neoadjuvant therapy in esophageal cancer was provided recently by a single-institution study from Spain. This was a prospective trial of neoadjuvant chemotherapy and concomitant chemoradiotherapy with cisplatinum (CDDP), 5 fluorouracil (5-FU), and 50.4 Gy of external radiotherapy before possible radical surgery in patients with locally advanced resectable esophageal cancer. A second-phase radiotherapy boost of 10 Gy and one cycle of modified-dose chemotherapy were used. Of 73 patients with predominantly (83%) squamous cell carcinoma and advanced locoregional disease (36% stage II, 54% stage III, and 47% local LN), 54% demonstated a response. Twenty-five of the patients proceeded to surgery, and radical resection was performed in 24. The complete pathological response rate was 32%. There were 7 postoperative deaths. Of the 34 patients who did not have surgery, 11 received the second-phase boost. Median OS was 10.3 months. The 2-year and 5-year OS were 22% and 16%, respectively. The only prognostic factor in OS was the clinical complete response rate: 13.9 versus 7.7 months (P=.0049).[21]
A recent meta-analysis showed a significant benefit for trimodality therapy in esophageal cancer. Eleven randomized controlled trials that included 1,308 patients showed that neoadjuvant chemoradiotherapy significantly improved OS compared with surgery alone. The odds ratio was 1.28 (P=.05) for 1-year survival, 1.78 (P=.004) for 3-year survival, and 1.46 (P=.02) for 5-year survival. Postoperative mortality was greater in the patients treated with neodjuvant chemoradiotherapy (P=.04), but postoperative complications were similar in the two groups. Neoadjuvant chemoradiotherapy lowered the incidence of locoregional cancer recurrence (P=.04); the incidence of distant cancer recurrence was similar in the two groups. Squamous cell carcinoma did not benefit from neoadjuvant chemoradiotherapy in this study: the odds ratio was 1.16 (P=.34) for 1-year survival, 1.34 (P=.07) for 3-year survival, and 1.41 (P=.06) for 5-year survival.[22]
The value of adding targeted therapy to treatment of esophageal cancer was discussed in a paper from the Cleveland Clinic. Gefitinib (Iressa) was added to concurrent chemoradiotherapy (CCRT) for locoregionally advanced esophageal/GEJ cancer to reduce distant metastases in patients with T3, N1, or M1a disease staged by EUS and PET/CT. A total of 80 patients were enrolled; they received continuous IV cisplatin (20 mg/m2/d) for 4 days and 5-FU (1000 mg/m2/d) on day 1 of preoperative radiation (30 Gy /1.5 Gy bid). Surgery followed in 4 to 6 weeks, and an identical course of CCRT was given 6 to 10 weeks postoperatively. Gefitinib, 250 mg/d, was given with preoperative CCRT for 4 weeks and restarted with postoperative therapy and continued for 2 years. Gefitinib did not increase toxicity except for the development of rash in 42 patients (53%) and diarrhea in 44 (55%). OS was improved (42% versus 28%, P=.06). Intolerance of gefitinib maintenance occurred in 48% of patients; those who experienced diarrhea appeared to have better outcomes.[23]
Although postoperative radiation for esophageal cancer is offered in selected cases, there is conflicting evidence as to whether it improves OS. Patients with T3-4,N0,M0 or T1-4,N1,M0 esophageal adenocarcinoma or squamous cell carcinoma who were definitively treated with esophagectomy, with or without postoperative radiation, were evaluated in a recent study. The 1046 study participants included 683 (65.3%) who were treated with surgery alone and 363 (34.7%) who also received postoperative radiation. There was significant improvement in median and 3-year OS (P<.001) and DSS (P<.001), for both stage III squamous cell carcinoma and stage III adenocarcinoma.[24]
Reference Guide
Therapeutic Agents
Mentioned in This Article
Capecitabine (Xeloda)
Cisplatin
Epirubicin
Fluorouracil
Gefitinib (Iressa)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
A phase III study by Stahl and colleagues[25] demonstrated that preoperative chemotherapy alone may not be an adequate approach for patients with resectable GEJ adenocarcinomas. A recent phase II study published by Starling and colleagues evaluated an approach in which patients received preoperative chemotherapy with epirubicin, cisplatin, and capecitabine (Xeloda).[26] The primary endpoint of the study was pathological complete response. The study was discontinued early because the primary endpoint had not been met at the time of the interim analysis. Of 28 evaluable patients, the response rate was estimated to be 46%. Subsequently, 76% of these patients underwent resection, with 73% undergoing an R0 resection and 27% undergoing R1 resection. The pathological complete response rate was only 5.9% in the intent-to-treat population. These results are similar to those obtained by others and indicate that for patients with distal esophageal or GEJ adenocarcinomas, the standard of care should be combined modality treatment when a neoadjuvant approach is pursued. If radiation therapy is not included preoperatively, then additional chemotherapy after resection should be administered.
Conclusion
The current standard of care for stage II and III esophageal cancer appears to be neoadjuvant chemoradiation consisting of a variety of chemotherapy regimens in conjunction with full-dose radiation therapy. This approach has made it possible to deliver the full dose of treatment up front and also results in a reasonable pathological complete response rate. A stage-specific approach to esophageal cancer can result in better outcomes.
Financial Disclosure:The author has no significant financial interests or other relationships with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Duan L, Wu AH, Sullivan-Halley J, et al. Passive smoking and risk of oesophageal and gastric adenocarcinomas. Br J Cancer. 2009;100:1483-85.
2. Lu XM, Monnier-Benoit S, Mo LZ, et al. Human papillomavirus in esophageal squamous cell carcinoma of the high-risk Kazakh ethnic group in Xinjiang, China. Eur J Surg Oncol. 2008;34:765-70.
3. Lagergren J, Ye W, Lagergren P, et al. The risk of esophageal adenocarcinoma after antireflux surgery. Gastroenterology. 2010;138:1297-1301.
4. Mezhir JJ, Coit DG, Jacks LM. Impact of positive peritoneal cytology on outcome in 291 patients with gastric cancer. Abstract presented at 2010 Gastrointestinal Cancers Symposium; 2010 Jan 22-24; Orlando, Fla.
5. Liptay MJ, D’Amico TA, Nwogu C, et al. Intraoperative SLN mapping in lung cancers. B J Thorac Oncol. 2010;4:198-202.
6. Grotenhuis BA, Wijnhoven BP, van Marion R, et al. The sentinel node concept in adenocarcinomas of the distal esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg. 2010;138:608-12.
7. Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009;22:1-8.
8. Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46-50.
9. Gaur P, Hofstetter W, Bekele BN, et al. Comparison between established and the worldwide esophageal cancer collaboration staging systems. Ann Thorac Surg. 2010;89:1797-1804.
10. American Joint Committee on Cancer. Edge SB, Byrd DR, Compton CC, et al, editors. Cancer staging manual. 7th ed. New York: Springer; 2010.
11. International Union Against Cancer. Staging malignant tumors. In: Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009.
12. Javeri H, Xiao L, Rohren E, et al. The higher the decrease in the standardized uptake value of positron emission tomography after chemoradiation, the better the survival of patients with gastroesophageal adenocarcinoma. Cancer. 2009;115:5184-92.
13. Bolton WD, Hofstetter WL, Francis AM, et al. Impact of tumor length on long-term survival of pT1 esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2009;138:831-6.
14. Brooke BS, Meguid RA, Makary MA, et al. Improving surgical outcomes through evidence based measures. Surgery. 2010;147:481-90.
15. Skipworth RJ, Parks RW, Stephens NA, et al. The relationship between hospital volume and post-operative mortality rates for upper gastrointestinal cancer resections Scotland 1982-2003. Eur J Surg Oncol. 2010;36:141-47.
16. Boudourakis LD, Wang TS, Roman SA, et al. Evolution of the surgeon-volume, patient-outcome relationship. Ann Surg. 2009;250:159-65.
17. Parameswaran R, Veeramootoo D, Krishnadas R, et al. Comparative experience of open and minimally invasive esophagogastric resection. World J Surg. 2009;33:1868-75.
18. Yannopoulos P, Theodoridis P, Manes K. Esophagectomy without thoracotomy: 25 years of experience over 750 patients. Langenbecks Arch Surg. 2009;394:611-6.
19. Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodality therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-7.
20. Tepper J, Krasna M, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-92.
21. Diaz R, Reynes G, Tormo A, et al. Long-term results of neoadjuvant chemotherapy and combined chemoradiotherpy before surgery in the management of locally advanced oesophageal cancer: a single-center experience. Clin Transl Oncol. 2009;11:835-41.
22. Jin HL, Zhu H, Ling TS, et al. Neoadjuvant chemoradiotherpy for resectable esophaegal carcinoma: a meta-analysis. World J Gastroenterol. 2009;15:5983-91.
23. Rodriguez CP, Adelstein DJ, Rice TW, et al. A phase II study of perioperative concurrent chemotherapy, gefitinib, and hyperfractionated radiation followed by maintenance gefitinib in locoregionally advanced esophagus and gastroesophageal junction cancer. J Thorac Oncol. 2010;5:229-35.
24. Schreiber D, Rineer J, Vongtama D, et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol. 2010;5:244-250.
25. Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851-6.
26. Starling N, Okines A, Cunningham D. A phase II trial of preoperative chemotherapy with epirubicin, cisplatin and capecitabine for patients with localised gastro-oesophageal junctional adenocarcinoma. Br J Cancer. 2009;100:1725-30.