Lung Cancer After 70: Is it a Different Disease?
Despite the fact that elderly patients comprise over 50% of the non-small cell lung cancer (NSCLC) population, our knowledge regarding the efficacy and safety of chemotherapy in this group is suboptimal. The “elderly” (defined as individuals ≥70 years of age) experience physiologically normal aging of their bone marrow and kidneys, which inherently increases toxicity to therapy. Standard practice has often been to discourage the use of combination chemotherapy in these patients; however, general consensus guidelines emphasize that performance status should primarily guide the choice of treatment. Elderly patients with advanced NSCLC treated with platinum doublet therapy demonstrate similar efficacy (but increased toxicity) to their younger counterparts. Patients with metastatic disease in which a targeted and/or biological agent(s) was added to chemotherapy experienced benefits similar to those treated with standard platinum doublets, but with increased morbidity and mortality. In the future, effective testing of molecular targeted therapies will have to include elderly patients among research cohorts or risk excluding a large population of eligible patients. Overall, elderly patients with advanced NSCLC, while experiencing greater toxicity, demonstrate the same response rates and survival benefits as their younger peers.
Despite the fact that elderly patients comprise over 50% of the non-small cell lung cancer (NSCLC) population, our knowledge regarding the efficacy and safety of chemotherapy in this group is suboptimal. The “elderly” (defined as individuals ≥70 years of age) experience physiologically normal aging of their bone marrow and kidneys, which inherently increases toxicity to therapy. Standard practice has often been to discourage the use of combination chemotherapy in these patients; however, general consensus guidelines emphasize that performance status should primarily guide the choice of treatment. Elderly patients with advanced NSCLC treated with platinum doublet therapy demonstrate similar efficacy (but increased toxicity) to their younger counterparts. Patients with metastatic disease in which a targeted and/or biological agent(s) was added to chemotherapy experienced benefits similar to those treated with standard platinum doublets, but with increased morbidity and mortality. In the future, effective testing of molecular targeted therapies will have to include elderly patients among research cohorts or risk excluding a large population of eligible patients. Overall, elderly patients with advanced NSCLC, while experiencing greater toxicity, demonstrate the same response rates and survival benefits as their younger peers.
Introduction
It is estimated that lung cancer will be diagnosed in 222,520 patients in 2010 and that 80% of them will fall into the non-small cell category (NSCLC).[1] Approximately 50% of these patients will be aged 65 years or older.[2] The SEER database indicates that the median age of diagnosis for NSCLC is 69 years of age, with 14% of newly diagnosed patients 80 years old or older-this latter group receives fewer interventions and shows poor survival.[3,4] Furthermore, the age-adjusted incidence of developing lung cancer of any type increases from one in 41 patients from 60 to 69 years of age to 1 in 18 patients at over 70 years of age according to data acquired between 2003 and 2005.[1] In the United States and Europe, 70 is accepted as “elderly”.[5] Patients ≥70 years of age are grossly underrepresented in clinical trials. Historically, only 25% of NSCLC trials included patients 65 years or older.[6] Extrapolating these data to elderly patients in clinical practice represents an obvious clinical dilemma.
However, age alone does not accurately predict patients’ ability to tolerate chemotherapy, and being elderly does not portend poor outcomes. Performance status (PS), rather than age, is a better predictor and should be used to tailor therapy decisions. Furthermore, multiple geriatric assessments have been constructed to better predict which elderly patients can tolerate chemotherapy. Questionnaires, such as ones developed by Hurria and Extermann et al, use a patient’s functional status, which combines nutritional status, mobility assessment, and concurrent health conditions to better predict for morbidity and mortality during treatment.[7,8]
In this article, we aim to demonstrate that elderly patients with advanced (stage III-IV) NSCLC will derive the same benefit from therapy as their younger age-matched controls. Topics to be addressed include toxicity, choice of single-agent or doublet chemotherapy, concurrent chemoradiotherapy, and finally choice of therapy in metastatic disease, with attention paid to toxicity of biological/targeted agents.
Pharmacology and Toxicity
Treatment of elderly cancer patients requires careful regard for potential toxicity. Normal aging results in a decrease in the glomerular filtration rate, which reduces the excretion of many common cytotoxic therapies used in NSCLC such as carboplatin and pemetrexed. The elderly must be careful to avoid dehydration, and nephrotoxic agents such as contrast dye, aspirin, or NSAIDs must be used with caution.[9, 10] Similarly, aging results in decreased hematopoietic progenitors and increased myelosuppression during chemotherapy.[11]
From 2003 to 2005, advanced-stage NSCLC patients were enrolled in the Cancer Care Outcomes Research and Surveillance [CanCORS] Consortium observational study[12]. Chrischilles et al prospectively evaluated a cohort of 1,371 of these NSCLC patients with age groups of <55, 55 to 64, 65 to 74, and ≥75 for the influence of age on toxicity and choice of therapy.[13] They found that 78% of patients in the <55 group received chemotherapy, while only 60.5% of those in the 65 to 74 group and 42% in the ≥75 group received any therapy (P < .0001). Similarly, older patients were less likely to receive platinum agents. Among those receiving chemotherapy, pre-treatment clinically important acute medical events (as defined in the CanCORS survey) actually occurred in 18.5% of patients age <55 years, but 9.2% of elderly patients. This is in contrast to treatment-related adverse events, which occurred in 42% of patients age 65-74 years, compared to 30.6% of patients <55, which was statistically significant (odds ratio: 1.70, 95% confidence interval: 1.19 to 2.43). Both peripheral neuropathy and neutropenic fever/sepsis occurrences were also associated with increasing age. Some reduction in the incidence of neutropenia may have been achieved via growth factor administration, per the guidelines recommended by the American Society of Clinical Oncology (ASCO).[14]
Doublet vs Single-Agent Therapy
The most recently published expert consensus data did not support the selection of a specific first-line chemotherapy regimen or combination therapy based on age alone, and in fact emphasized that elderly patients derive the same benefit as younger groups, but at the cost of increased toxicity with combination versus single agent first-line chemotherapy.[15, 16]
In the West Japan Thoracic Oncology Group (WJTOG) 9904 trial, in which docetaxel was compared with vinolrelbine in elderly patients with advanced-stage disease (including PS ≤2), docetaxel (5.5 vs 3.1 months, P < .001, and 22.7% vs 9.9%, P < .01, respectively) showed a higher progression-free survival (PFS) and response rate, but also showed higher myelosuppression.[17]
Docetaxel has also been compared to a combination of gemcitabine-docetaxel in patients >65 years of age. In the combination arm, 39% of patients were >75 years. Time to progression was improved with the combination therapy (4.8 vs 2.9 months; P = .04), but overall survival (OS) results showed no statistical difference. Those with Eastern Cooperative Oncology Group (ECOG) PS of 2 did poorly, and the authors suggested that PS should influence the choice of singlet vs doublet therapy.[18]
TABLE 1
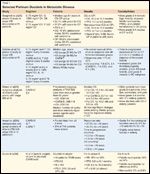
Selected Platinum Doublets in Metastatic Disease
A pivotal trial conducted by Quoix et al was recently presented in abstract form (Table 1); this study intended to show that doublet therapy is superior to singlet therapy in the eldest of patients.[19] The trial included only patients aged 70 to 89 years; in addition, patients with ECOG PS of 2 comprised ~27% of the total study population (n = 451). Patients were randomized to 15 weeks of either single-agent weekly gemcitabine or vinorelbine or to a combination of carboplatin, dosed monthly, with weekly paclitaxel for a total of 16 weeks. Doublet therapy demonstrated an improvement in PFS from 3.0 to 6.1 months for singlet therapy (P < 10-6). OS for doublet therapy was 10.3 months, vs 6.2 months in the single-agent arm (P < .00004). Grade 3 neutropenia occurred in 54% of patients who received doublet therapy and in 38% of those who received singlet therapy (P < 10-5), but no significant differences were shown regarding febrile neutropenia. Toxic deaths were increased with doublet therapy, but overall death rates were higher in the singlet therapy group.
Overall, this trial provides level I evidence for using PS criteria and not age as the most important factor in choosing doublet chemotherapy, since older patients with good PS achieve gains, similar to those seen in their younger counterparts, in PFS and OS with this therapy. This trial provides level I evidence to the ASCO consensus guidelines, which recommend consideration of doublet therapy in fit elderly individuals.[15]
Choice of Doublet Therapy
In the landmark ECOG 1594 study, three cisplatin-based doublets (gemcitabine, docetaxel, paclitaxel) and carboplatin-paclitaxel were evaluated in the front-line setting for advanced NSCLC[20]. A total of 1,155 eligible patients were enrolled, with a median age of 63 years. The median OS for all groups was 8.0 months, with a one-year survival rate of 34% and a two-year survival rate of 12%. No significant differences in OS and response rates were found between the groups. Although time to progression was improved with cisplatin-gemcitabine vscisplatin-paclitaxel (4.2 months vs 3.4 months), it occurred at the expense of more grade 4 platelet toxicity and grade 4/5 renal toxicity.
An age-based subgroup analysis was not performed in this study, but patients with ECOG PS of 2 demonstrated high rates of adverse events and were later excluded from eligibility to the study. The median OS for these patients was 3.9 months. compared to 10.1 and 7.1 months for patients with better PS (ECOG PS 0 and 1, respectively). Carboplatin-paclitaxel was chosen as the future ECOG reference regimen, due to its comparable activity (survival) and better safety (less toxicity) versus the other regimens tested (Table 1).
Belani and Fossella evaluated efficacy and toxicity of cisplatin-docetaxel, carboplatin-docetaxel, and cisplatin-vinorelbine in patients ≥65 in a subgroup analysis of the TAX 326 trial.[21] Of the 1,218 patients enrolled, 33% were >65 years of age. Median survival was 12.6 months in the docetaxel-cisplatin arm, compared to 9.9 months in the vinorelbine-cisplatin arm and 9.0 months in the docetaxel-carboplatin arm. Elderly patients tolerated chemotherapy relatively well but experienced increased neurotoxicity, asthenia, pulmonary toxicity, diarrhea (8.1% vs 5.8%), neutropenia (81.8 vs 70.7%), and febrile neutropenia (8.1% vs 3.1%).
Clinically speaking, patients in the TAX 326 trial and other trials may have better tolerance if therapy is dosed weekly, as described elsewhere,[22, 23] this is possible because weekly dosing may allow for more frequent surveillance of toxic adverse effects, dehydration, and infections during therapy (Table 1).
Ansari et al presented a study in which a retrospective subgroup analysis of the elderly (>70 years old) was performed on a randomized phase III study comparing carboplatin-gemcitabine, carboplatin-paclitaxel, and gemcitabine-paclitaxel doublets in metastatic disease.[24] Data were pooled from all three treatment arms to evaluate efficacy and toxicity in older versus younger patients. No significant differences were found in response rates, PFS, or OS. Slightly more hematologic toxicity was reported in the ≥70 population (Table 1).
The introduction of pemetrexed-based combination chemotherapy has altered standard practice for first-line therapy. In 2008, Scagliotti et al compared cisplatin-pemetrexed vs cisplatin-gemcitabine in a randomized phase III study of patients with PS 0-1 receiving first-line treatment for metastatic or unresectable NSCLC.[25] The median age in the study was 61 years; 37.2% of cisplatin-pemetrexed and 33.1% of cisplatin-gemcitabine patients were ≥65 years. In a pre-planned analysis, the use of cisplatin-pemetrexed in non-squamous histology was associated with a statistically significant improvement in both OS and PFS compared to cisplatin-gemcitabine (11.8 vs 10.4 months and 5.3 vs 4.7 months, respectively). This study noted the importance of histology when selecting pemetrexed-based therapy and compared favorably with historical controls with respect to OS.
In patients ≥65 years old, the survival hazard ratio trended in favor of cisplatin-pemetrexed (0.88). Significantly more grade 3/4 hematologic, febrile neutropenia, alopecia, and red blood cell and platelet transfusion toxicities were seen in the cisplatin-gemcitabine group. More grade 3/4 nausea was seen in the cisplatin-pemetrexed group. In summary, cisplatin-pemetrexed demonstrated improved OS and PFS in non-squamous histology patients, with less toxicity. There was a trend toward increased survival in the elderly (Table 1). Updated survival data has resulted from pemetrexed maintenance therapy given after induction platinum doublet chemotherapy, and this has taken advanced NSCLC survival to new levels. The OS for the entire cohort of patients in this study was 13.4 months, and the number was even higher in the subgroup of patients with adenocarcinoma histology.[26]
Overall, the use of any platinum doublet to treat advanced NSCLC shows similar efficacy, but with slight differences in toxicity. Prior to the introduction of pemetrexed, which is more effective in non-squamous histologies, no specific clinical characteristic would aid in the selection. Together, the above-mentioned studies continue to reveal an ongoing theme, demonstrated mostly with post hoc analysis, retrospective subgroup analyses, and rarely in randomized trials: Elderly patients have similar levels of response rates and OS, but added toxicity, particularly myelosuppression.
Physicians who treat adenocarcinoma and non-squamous histology have added options to their treatment regimens that include pemetrexed therapy. Whether the survival benefit from pemetrexed is best achieved via usage as a front line, second line, or maintenance is yet to be determined. This remains a viable and relatively well-tolerated agent for elderly patients who require treatment for advanced-staged disease. The choice of therapy for advanced NSCLC with squamous histology still remains difficult as our therapeutic armamentarium is limited, regardless of age. Reasonable options include platinum/taxane combinations or combinations with gemcitabine in this group of squamous histology patients.
Concurrent Chemoradiotherapy
In patients with unresectable stage III NSCLC, the treatment of choice has often been combined modality chemoradiotherapy. As in the past, the most current ASCO guidelines suggest that a concurrent chemoradiotherapy approach be reserved for patients with good PS.[15] As clinicians, we are all well aware of the toxicities, particularly esophagitis, pneumonitis, and myelosuppression, that are related to concurrent chemotherapy and how they contribute to morbidity and mortality of patients, even in our youngest and fittest patients.
TABLE 2

Concurrent Chemoradiotherapies
There are no prospective randomized trials that have evaluated chemoradiotherapy in elderly patients. Prospective trials that include elderly patients and that were designed to evaluate the role of combined therapies commonly employed in clinical practice are detailed in Table 2. Modifications in practice of the regimens listed in Table 2 often include weekly dosing, substitution of cisplatin for carboplatin, and reduction in total days of etoposide administered.[27]
Studies completed by Albain et al and Ganadra et al, which utilized cisplatin-etoposide concurrent with thoracic radiation, included only patients with ECOG PS of 0 or 1 and limited numbers of elderly patients. Although these Southwest Oncology Group regimens have shown impressive median OS data of 26 months, the resulting short-term hematological and non-hematological toxicities may outweigh the benefits in less-fit patients.[28,29]
There are several retrospective subgroup studies taken from randomized phase III trials that evaluated concurrent chemoradiotherapy between patients ≥70 years old and those <70 years old, with only one trial (Sgroi et al) evaluating consolidation chemotherapy.[30-32]. Survival results were similar in elderly and younger patients; however, the former experienced increased esophagitis, myelosuppression, and dehydration and a higher rate of early treatment termination. Altogether, combining the previous prospective phase II data (Table 1) and the hypothesis-generating data from these retrospective reviews, we can clearly establish that elderly patients must have their therapies tailored to PS with careful observation and quick intervention for the increased toxicity they will experience during chemoradiotherapy.
Targeted Therapies for Metastatic Disease
In the ECOG 4599 randomized phase III trial, the group assigned to paclitaxel-carboplatin (PC) plus bevacizumab (PCB) demonstrated a median survival of 12.3 months compared with 10.3 months in the chemotherapy-alone (PC) group (hazard ratio for death: 0.79; P = .003).[33] A subgroup analysis of this study evaluated 224 patients aged ≥70 (26%) from the total 878 initial trial participants.[34] In terms of response and PFS, elderly patients treated with PCB (compared to PC alone) showed a trend toward higher response rates (29% vs 17%; P = .067) and PFS (5.9 vs 4.9 months; P = .063). There was no difference in OS (11.3 vs 12.1 months; P = .40); however, this subgroup analysis was not designed to detect such a difference. Elderly patients had higher incidence of grade 3-5 adverse events in the PCB than in the PC alone arm (87% vs 61%; P < .001). Neutropenic fever rate was 6.2% (PCB) vs 0.9% (PC) (P = .03). Elderly patients on the PCB arm also had more bleeding, anorexia, and nausea than did younger patients. Most importantly, among elderly patients there were seven treatment-related deaths on PCB, versus only two deaths on the PC alone arm. Further study is warranted in order to understand which elderly patients will benefit from, and which will be harmed by bevacizumab.
The Canadian BR.21 study evaluated erlotinib versus placebo in the second line and reviewed outcomes of 163 (22%) elderly patients (age ≥70 years) out of 731 total patients.[35] There were no significant differences between elderly and non-elderly patients regarding PFS (3.0 vs 2.1 months for erlotinib vs placebo; P = .009), response rates, and OS (7.6 vs 5.0 months in elderly [P = .67] and 6.4 vs 4.7 months in young patients [P = .0014] for erlotinib vs placebo, respectively). Elderly patients also derived similar quality-of-life improvements from treatment. Regarding toxicity, more elderly patients had grade 3/4 toxicity (35% vs 18%; P < .001), and more elderly patients were required to stop treatment because of these toxicities (12% vs 3%; P < .001). Rash and diarrhea were the most common adverse events. Thus, erlotinib remains a viable treatment option among elderly patients for second-line therapy.
Conclusions
NSCLC is becoming increasingly common among our elderly population. The most important factor in choosing a patient’s initial and subsequent chemotherapy is PS, not age. We obviously must be cautious in our choice of therapies, as decreasing bone marrow and kidney reserve, hearing loss, reduced hepatic synthetic function, and corresponding tobacco-related pulmonary and cardiovascular complications can lead to increased therapy-related toxicities in the elderly population. In one of the few randomized trials performed in elderly patients with advanced NSCLC, Quoix et al clearly demonstrated a survival benefit for doublet chemotherapy with carboplatin and paclitaxel over single-agent gemcitabine or vinorelbine.[18] Therefore, offering single-agent therapy because of age alone is no longer justified. Prior to the introduction of pemetrexed for non-squamous histologies, no survival benefit existed among platinum doublets for advanced disease; however, elderly patients consistently suffered increased toxicity. The use of pemetrexed, when appropriate, offers improved survival and less toxicity, a benefit that is retained among the elderly. There is no standard of care for concurrent chemoradiotherapy in this population.
A concerning and noteworthy observation that has come from retrospective subgroup analyses is that grade 5 events associated with bevacizumab have been observed in the elderly. The toxicity profile of different biologic agents in the elderly is a field of great interest that requires further study both clinically and in the laboratory.
The efficacy of individualized cancer care-based on a patient’s tumor genetics and mutational status-with agents such as erlotinib and other targeted therapies has yet to be evaluated in this elderly population. Cost effectiveness also becomes a question as we look toward an era in which EGFR, KRAS, ALK, and others, are evaluated during diagnosis. Oncologists and insurers will have to balance their excitement over novel discoveries with a rational assessment of the level of tolerance they see when implementing new treatments in our elderly population. Advanced NSCLC is the same disease in young or old, and treatment decisions must be based on PS and comorbidities generating specific pharmacologic contraindications. All oncologists must discuss the risks of increased toxicity with their elderly patients and caregivers and inform them of how to best deal with the potential complications, should they arise.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Acknowledgments: We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
References:
References
1. Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225-49.
2. Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11:437-41.
3. Havlik RJ, Yancik R, Long S, et al. The National Institute on Aging and the National Cancer Institute SEER collaborative study on comorbidity and early diagnosis of cancer in the elderly. Cancer. 1994;74:2101-6.
4. Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570-7.
5. Balducci L. Geriatric oncology: challenges for the new century. Eur J Cancer. 2000;36:1741-54.
6. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061-7.
7. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824-31.
8. Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104:1998-2005.
9. Himmelfarb J. Acute kidney injury in the elderly: problems and prospects. Semin Nephrol. 2009;29:658-64.
10. Rosner MH. The pathogenesis of susceptibility to acute kidney injury in the elderly. Curr Aging Sci. 2009;2:158-64.
11. Gazit R, Weissman IL, Rossi DJ. Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol. 2008;45:218-24.
12. Chrischilles EA, Pendergast JF, Kahn KL, et al. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:620-7.
13. Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187-205.
14. Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187-205.
15. Azzoli CG, Baker S, Jr, Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol. 2009;27:6251-66.
16. Pallis AG, Gridelli C, van Meerbeeck JP, et al. EORTC Elderly Task Force and Lung Cancer Group and International Society for Geriatric Oncology (SIOG) experts’ opinion for the treatment of non-small-cell lung cancer in an elderly population. Annals of Oncology. 2010;21:692-706.
17. Kudoh S, Takeda K, Nakagawa K, et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol. 2006;24:3657-63.
18. Hainsworth JD, Spigel DR, Farley C, et al. Weekly docetaxel versus docetaxel/gemcitabine in the treatment of elderly or poor performance status patients with advanced nonsmall cell lung cancer: a randomized phase 3 trial of the Minnie Pearl Cancer Research Network. Cancer. 2007;110:2027-34.
19. Quoix EA, Oster J, Westeel V, et al. Weekly paclitaxel combined with monthly carboplatin versus single-agent therapy in patients age 70 to 89: IFCT-0501 randomized phase III study in advanced non-small cell lung cancer (NSCLC). J Clin Oncol (Meeting Abstracts). 2010;28:2-.
20. Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92-8.
21. Belani CP, Fossella F. Elderly subgroup analysis of a randomized phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for first-line treatment of advanced nonsmall cell lung carcinoma (TAX 326). Cancer. 2005;104:2766-74.
22. Ramalingam S, Barstis J, Perry MC, et al. Treatment of elderly non-small cell lung cancer patients with three different schedules of weekly paclitaxel in combination with carboplatin: subanalysis of a randomized trial. J Thorac Oncol. 2006;1:240-4.
23. Ramalingam S, Perry MC, La Rocca RV, et al. Comparison of outcomes for elderly patients treated with weekly paclitaxel in combination with carboplatin versus the standard 3-weekly paclitaxel and carboplatin for advanced nonsmall cell lung cancer. Cancer. 2008;113:542-6.
24. Ansari R GR, Socinski M et al., editor. Elderly subgroup analysis of a randomized phase 3 trial of gmcitabine in combiantion with carboplatin or paclitaxel compared to paclitaxel plus carboplatin in advanced non-small cell lung cancer. Proc Am Soc Clin Oncol 2007.
25. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543-51.
26. Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432-40.
27. Stinchcombe TE, Gore EM. Limited-stage small cell lung cancer: current chemoradiotherapy treatment paradigms. Oncologist. 2010;15:187-95.
28. Albain KS, Crowley JJ, Turrisi AT, 3rd, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002;20:3454-60.
29. Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21:2004-10.
30. Rocha Lima CM, Herndon JE, 2nd, Kosty M, et al. Therapy choices among older patients with lung carcinoma: an evaluation of two trials of the Cancer and Leukemia Group B. Cancer. 2002;94:181-7.
31. Schild SE, Stella PJ, Geyer SM, et al. The outcome of combined-modality therapy for stage III non-small-cell lung cancer in the elderly. J Clin Oncol. 2003;21:3201-6.
32. Sgroi MM, Neubauer M, Ansari R, et al. An analysis of elderly patients (pts) treated on a phase III trial of cisplatin (P) plus etoposide (E) with concurrent radiotherapy (CRT) followed by docetaxel (D) vs observation (O) in pts with stage III non small cell lung cancer (NSCLC). J Clin Oncol (Meeting Abstracts). 2007;25:9037-.
33. Sandler A, Gray R, Perry MC, et al. Paclitaxel-Carboplatin Alone or with Bevacizumab for Non-Small-Cell Lung Cancer. N Engl J Med. 2006;355:2542-50.
34. Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26:60-5.
35. Wheatley-Price P, Ding K, Seymour L, et al. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26:2350-7.
36. Belani CP, Choy CP, Choy H, et al. Combined chemoradiaotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883-91.