New Potential Frontline Standards for RCC Are Spotlighted at Genitourinary Cancers Symposium
ONCOLOGY reviews key updates in the treatment of renal cell carcinoma to come out of the Genitourinary Cancers Symposium.
Combination therapy coupling immunotherapy with tyrosine kinase inhibitors (TKIs) with activity against VEGF receptors for the treatment of frontline renal cell carcinoma (RCC) took center stage at the 2021 Genitourinary Cancers Symposium, an event cosponsored by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology.
Namely, the readout of the phase 3 CLEAR trial (NCT02811861) demonstrated that combining lenvatinib (Lenvima) with either pembrolizumab (Keytruda) or everolimus (Afinitor) improved progression-free survival (PFS) and objective response rate (ORR) over sunitinib (Sutent) for the treatment of patients with advanced RCC receiving therapy in the frontline setting.
Additionally, the combination with pembrolizumab was able to improve overall survival (OS) versus the control group in this patient population. These data were simultaneously presented virtually during the Genitourinary Cancers Symposium and published in The New England Journal of Medicine.1,2
“I think that lenvatinib [plus] pembrolizumab should be considered a standard treatment program. This awaits regulatory review and hopefully approval, but certainly from looking at the data, this is a highly effective [treatment regimen],” said Robert J. Motzer, MD in an interview with ONCOLOGY®. At Memorial Sloan Kettering Cancer Center in New York, he is head of the kidney cancer section, Genitourinary Oncology Service, and holds the Jack and Dorothy Byrne Chair in Clinical Oncology. “Safety is manageable. It’s a very attractive program for first-line therapy for clear cell RCC,” Motzer said.
Another immunotherapy/VEGF-targeting combination, nivolumab (Opdivo) plus cabozantinib (Cabometyx), continued to demonstrate promise according to quality-of-life (QOL) and subgroup analyses from the pivotal phase 3 CheckMate 9ER trial (NCT03141177). This led to the FDA approval of the combination in the frontline setting for RCC in January 2021.3
CLEAR Study
The CLEAR trial included 1069 patients with advanced, treatment-naïve RCC who were randomized 1:1:1 to receive daily oral lenvatinib at 20 mg plus intravenous pembrolizumab at 200 mg every 3 weeks (n = 355); daily oral lenvatinib at 18 mg oral plus everolimus at 5 mg (n = 357); or daily oral sunitinib at 50 mg for 4 weeks on and 2 weeks off (n = 357). PFS by independent review committee (IRC) per RECIST 1.1 was the primary end point; secondary end points included OS, ORR by IRC per RECIST 1.1, safety, and health-related QOL (HRQOL).
The median PFS with lenvatinib/pembrolizumab was 23.9 months versus 9.2 months with single-agent sunitinib (HR, 0.39; 95% CI, 0.32-0.49; P < .001). The lenvatinib/everolimus treatment arm achieved a median PFS of 14.7 months compared with 9.2 months in the sunitinib arm (HR, 0.65; 95% CI, 0.53-0.8; P < .001) (Table).1,2 The PFS benefit extended across all patient subgroups in both active therapy treatment arms.
TABLE. Median PFS and OS Across CLEAR Trial Active Therapy Arms
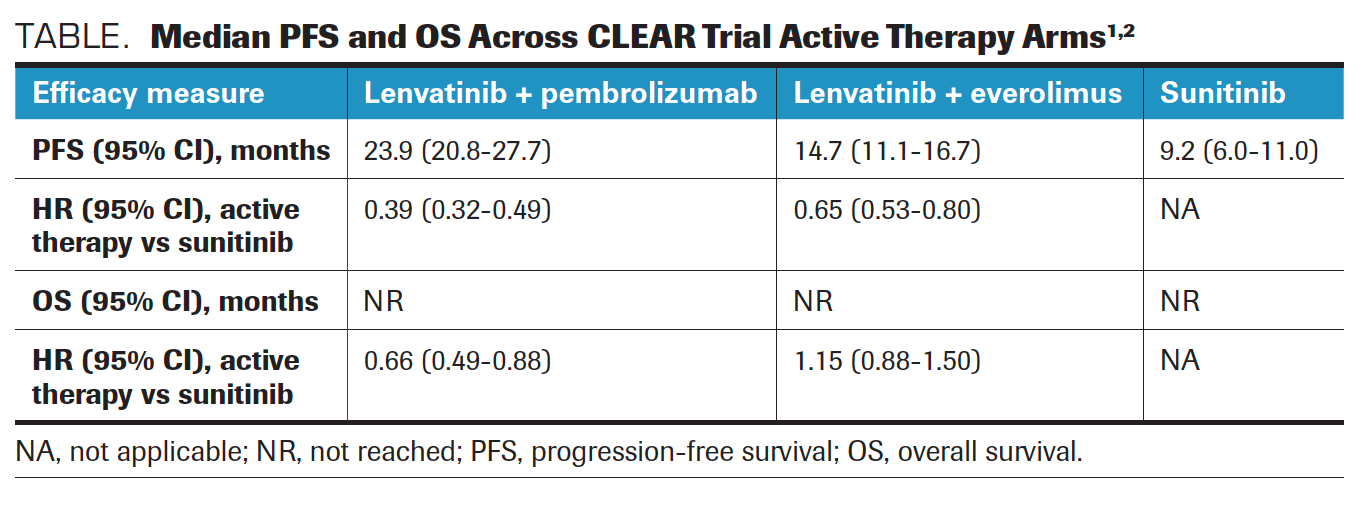
“The results of the analysis [were] really striking. The primary end points were PFS for both lenvatinib and pembrolizumab [as well as] lenvatinib and everolimus vs sunitinib, and the trial met the primary end point for each of those arms showing a longer PFS compared with sunitinib,” Motzer said.
A median OS was not reached in any of the 3 treatment arms; however, the data indicated that OS was significantly longer in the lenvatinib/pembrolizumab arm compared with the sunitinib arm (HR, 0.66; 95% CI, 0.49-0.88; P = .005). Motzer noted that there was no observed OS benefit with lenvatinib/everolimus over sunitinib alone (HR, 1.15; 95% CI, 0.88-1.50; P = .3).
ORR was higher in both the lenvatinib/pembrolizumab (71%; 95% CI, 66.3%-75.7%) and lenvatinib/everolimus (53.5%; 95% CI, 48.3%-58.7%) treatment arms compared with sunitinib (36.1%; 95% CI, 31.2%-41.1%). Of note, Motzer mentioned the “high complete response rate” of 16.1% in the lenvatinib-plus-pembrolizumab arm.
Patients in the lenvatinib/pembrolizumab arm achieved the longest median duration of response at 25.8 months, compared with 16.6 months and 14.6 months in the lenvatinib/everolimus and sunitinib arms, respectively.
Motzer noted that the safety profiles of both combinations were consistent with the known safety profile of each drug, and toxicities were manageable as needed for dose modifications.
CheckMate 9ER
According to extended follow-up data from CheckMate 9ER, nivolumab plus cabozantinib continued to demonstrate superior efficacy to single-agent sunitinib in the frontline treatment of patients with advanced RCC, and an exploratory subgroup analysis suggested that these survival and response benefits were seen regardless of sarcomatoid histology.4
Among patients with sarcomatoid features, the median PFS was 10.3 months in the combination arm vs 4.2 months with sunitinib monotherapy (HR, 0.42; 95% CI, 0.23-0.74). Patients who did not have sarcomatoid histology, on the other hand, had a median PFS of 17.5 months and 9.2 months in the doublet and single-agent arms, respectively (HR, 0.56; 95% CI, 0.45-0.69).
In terms of OS, the median was not reached in patients with or without sarcomatoid features who were treated with nivolumab/cabozantinib, nor was it reached for patients without sarcomatoid histology who were treated with sunitinib. The hazard ratio for OS in the sarcomatoid-positive group was 0.36 (95% CI, 0.17-0.79) and 0.73 (95% CI, 0.54-0.98) in the sarcomatoid-negative group.
Additional data from CheckMate 9ER found that patients with advanced RCC reported improved HRQOL when treated with nivolumab plus cabozantinib compared with treatment with sunitinib.5
Moreover, patients treated with the combination experienced a delay in deterioration and a significant decreased risk for confirmed deterioration in HRQOL scores, including disease-related kidney cancer symptoms.
Moving Forward
With these new data regarding immunotherapy combinations in the treatment of frontline RCC, key stakeholders who were in attendance at the meeting seemed optimistic about what these results mean going forward.
“We came all the way from single-agent [TKIs] to immunotherapy single agents; now we’re combining [them and] patients with metastatic RCC are living way longer, years and years on average,” said Toni Choueiri, MD, director of the Lank Center for Genitourinary (GU)
Oncology at Dana-Farber Cancer Institute/Brigham and Women’s Hospital as well as the Jerome and Nancy Kohlberg Chair and professor of medicine at Harvard Medical School in Boston, speaking in an interview about all the new data to come out of the conference.
“Some other studies are moving forward. Another combination trial is being planned—a large phase 3 trial that’s going to be using lenvatinib [plus] pembrolizumab as the standard arm for other combinations,” Motzer concluded. “I see this as setting a new standard, and hopefully [it] will also receive regulatory approval and be available for patients outside of studies.”
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
REFERENCES
1. Motzer RJ, Porta C, Eto M, et al. Phase 3 trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) monotherapy as a first-line treatment for patients (pts) with advanced renal cell carcinoma (RCC) (CLEAR study). J Clin Oncol. 2021;39(suppl 6):269. doi: 10.1200/JCO.2021.39.6_suppl.269
2. Motzer R, Alekseev B, Rha S-Y, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. Published online February 13, 2021. doi:10.1056/NEJMoa2035716
3. FDA approves nivolumab plus cabozantinib for advanced renal cell carcinoma. FDA. January 22, 2021. Accessed February 12, 2021. https://bit.ly/3iEIlMj
4. Motzer RJ, Choueiri TK, Powles T, et al. Nivolumab + cabozantinib (NIVO+CABO) versus sunitinib (SUN) for advanced renal cell carcinoma (aRCC): outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. J Clin Oncol. 2021;39(suppl 6):308. doi:10.1200/JCO.2021.39.6_suppl.308
5. Cella D, Choueiri T, Blum S, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with first-line nivolumab plus cabozantinib versus sunitinib: the CheckMate 9ER trial. J Clin Oncol. 2021;39(suppl 6):285. doi:10.1200/JCO.2021.39.6_suppl.285
