How to Radiologically Assess and Follow Response After Treatment of Hepatocellular Carcinoma
A thought-provoking installment of Clinical Quandaries is presented by Alejandro Gabutti, MD; and Tommaso Cascella, MD, of a 36-year-old patient with hepatitis C virus-related cirrhosis and a subsequent diagnosis of hepatocellular carcinoma.
Oncology (Williston Park). 2021;35(4):199-204.
DOI: 10.46883/ONC.2021.3504.0199
Gabutti is affiliated with the Department of Radiology at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City, Mexico.

Cascellais affiliated with the Department of Radiology at Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy.

A 6-cm liver tumor in segment 8 was found in a woman, aged 36 years, with hepatitis C virus (HCV)–related cirrhosis. The tumor was initially noticed in a screening ultrasound and further characterization was done with a multiphase CT scan. The tumor showed arterial enhancement and venous washout, and hepatocellular carcinoma (HCC) was definitively diagnosed.
For final staging, a chest CT and bone scintigraphy were performed. There was no evidence of extrahepatic disease or vascular invasion. At diagnosis, liver function was well compensated (Child-Pugh A5) and MELD (Model for End-Stage Liver Disease) score was 12. Alfa-fetoprotein plasma level was 7 ng/ml (within normal limits). Also, imaging studies revealed spleen enlargement and collateral venous circulation due to portal hypertension. The patient was classified as stage B according to the Barcelona criteria.
The patient’s relevant clinical history included sofosbuvir/velpatasvir (Epclusa) as treatment for HCV, which had been successfully completed 15 months before the HCC diagnosis. A sustained viral response was achieved.
Two weeks after diagnosis, the patient underwent a conventional transarterial chemoembolization (ethiodized oil [Lipiodol] plus doxorubicin, and polyvinyl alcohol [PVA] particles as embolic agent). The first follow-up was done with a multiphase CT 1 month after transarterial chemoembolization. No arterial enhancement was noticed, but there was a 2-cm area of absent ethiodized oil uptake within the tumor. Because of a high suspicion of tumor persistence, MRI was immediately performed, which showed a 1.8-cm viable residual tumor. A bland embolization with PVA particles was performed because of a doxorubicin shortage. Stable disease was documented. Due to residual tumor size and lack of response to transarterial embolization, a percutaneous ablation was performed. Because of the proximity of the tumor to the diaphragm, chemical ablation with ethanol was employed. Four weeks post treatment, an MRI showed a complete response.
What posttreatment follow-up should be done after achieving complete response?
Discussion
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a major global health concern.1 Its incidence has been rising over the last 20 years and is expected to increase until 2030.2 Diagnosis, treatment, and follow-up rely mainly on imaging. Thorough radiological assessment is critical in terms of satisfactory patient management.
HCC treatment must be decided by a multidisciplinary liver tumor board. Medical oncologists play an essential role, especially in patients with intermediate- or advanced-stage disease. Oncologists view the disease as a systemic issue, and along with clinical evaluation, radiological evaluations are probably the most important tools to promote the best outcomes.
The therapeutic armamentarium for HCC is broad and heterogeneous and can be divided into treatments with curative and noncurative intent.3 Curative treatments include liver transplantation, resection, and ablation. Noncurative treatments include transarterial chemoembolization (TACE) and variants, transarterial radioembolization (TARE), stereotactic body radiation therapy (SBRT), and systemic therapies.
FIGURE 1
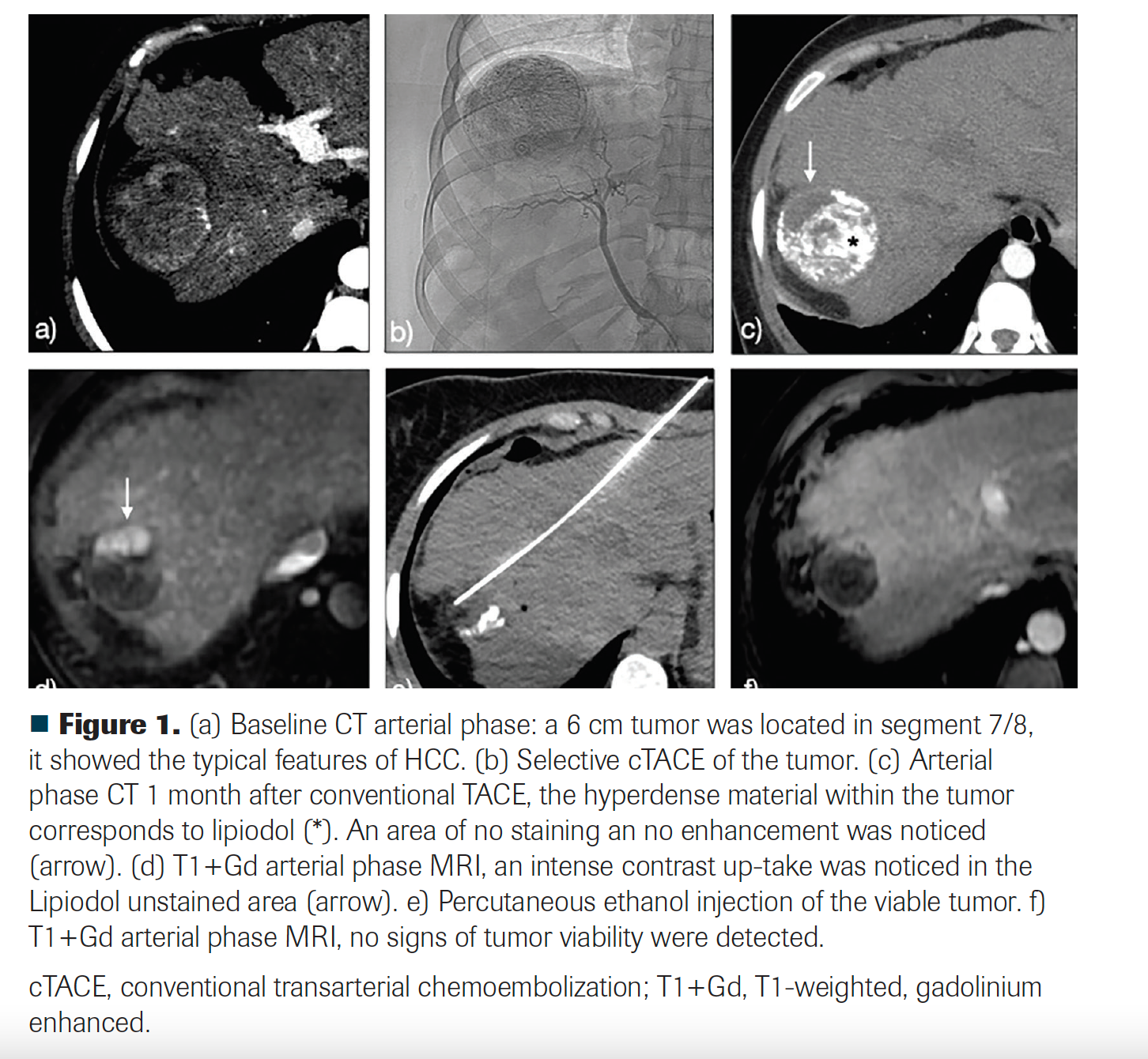
HCC treatments are under continuous development and evolution. New therapeutic strategies, including novel antiangiogenic agents, immunomodulators, and combinations with locoregional therapies (LRTs), are currently in the spotlight.4 An interesting example is the evolution of TARE. Technical improvements have transformed it to a potentially curative therapy when was it was previously considered a palliative treatment.5
How should we assess the response of HCC to treatment? This is a matter of constant debate and discussion.6
The radiological evaluation protocols after treatment are heterogeneous and may vary by institution and therapeutical strategy.7 The follow-up protocol strength relies on 3 essential points: timing, imaging method, and the chosen radiological response criteria. It is not the aim of this article to deeply review the imaging findings after locoregional or systemic therapies, but rather to review the evidence of when and how to assess response.
When to Assess Response After Treatment
The accepted timing for performing the first imaging study after treatment (LRT or systemic) is 4 to 6 weeks.7-9 An exception is when the patient has been treated with TARE or SBRT. The first imaging evaluation after a radiation-based LRT, unlike other LRTs, is 3 months.10 This is because radiation-induced tumor necrosis is often delayed.10-12 Once 4 to 6 weeks have elapsed, most of the inflammatory changes related to LRTs have resolved. However, inflammation and tumor persistence may be challenging to distinguish with imaging, as both usually appear as areas of arterial hyperenhancement on multiphase CT or MRI.13,14
The first imaging surveillance has 2 main objectives: to identify tumoral viability and early complications.8,15 If viable tumor is detected on the first imaging assessment after treatment, it is classified as residual disease.16 Large areas of residual tumor will be easily seen on the first imaging surveillance. Viable tumor shows the characteristic radiological appearance of HCC, especially arterial enhancement.17 However, this is not always obvious in small foci of residual tumor, and in such cases diagnosis can be challenging. Not uncommonly, a final diagnosis of tumor viability is made only on further follow-up.
If equivocal findings of residual tumor are detected initially, the imaging evaluation should be repeated no longer than 3 months after treatment (2 months after the first assessment) until a final diagnosis is established.12,18 The identification of viable tumor within the first follow-up must lead to a case reevaluation to guide further therapy.13,18,19
Other than detecting tumoral persistence, the first imaging evaluation is also important for early recognition of treatment-related complications, including necrosis infection, biloma formation, bile duct stricture, vascular injuries, or nontarget treatment effects.8,19 While the rate of complications after LRTs is low, typically less than 5%,20 early management of any that are detected is essential to avoid delay in future treatments.
The first radiological assessment can be focused just on local tumor response. Whole-body imaging is not necessary, unless metastatic disease was present at diagnosis. Once there is no evidence of tumor viability, an active surveillance protocol should be begun. Beyond this point, tumoral viability detected within a treated tumor is classified as recurrence.16
In early-stage HCC, when a curative-intent treatment (ablation or resection) was employed, patients can be monitored every 3 to 4 months during the first 2 years, with surveillance every 6 months thereafter.1,2,7 European Association for the Study of the Liver (EASL) guidelines emphasize particularly close follow-up of patients who also had successfully completed hepatitis C virus treatment with direct-acting antivirals.1
The every-6-months monitoring schedule should continue indefinitely. The majority of HCC cases develop in the context of cirrhosis. Therefore, the surveillance aim is not only the detection of late-onset recurrence, but also to recognize neohepatocarcinogenesis.
For patients with intermediate-stage disease, who are usually treated with embolotherapies, follow-up can be every 3 months.2 For patients with advanced-stage disease treated with systemic agents, a radiological evaluation every 2 to 3 months is suggested.7 Both schedules aim to monitor for tumor progression to guide future therapeutic decisions.2,7 The follow-up protocol should continue indefinitely or until there is a major management change, eg, successfully downstaging with subsequent liver transplantation as the final therapy.
How to Assess Response After Treatment
Two questions may arise in assessing response: First, in which situations should extraabdominal imaging studies be performed? And second, what is the best imaging method for evaluating local response after treatment? While there is lack of consensus or precise recommendations about these issues, certain facts support our recommendations, we believe.
Some clues in answering both questions are provided by the initial disease staging. The HCC work-up typically includes state-of-the-art liver imaging (multiphase CT or MRI), chest CT, and a bone scan.1,2 However, the usefulness of bone scan with technetium-99m methyl diphosphonate is controversial. Some authors suggest that in early-stage HCC (within Milan criteria), it is not cost-effective and should not be done.21,22
Even when the disease is radiologically limited to the liver, high tumor burden (index tumor > 5 cm), elevated levels of alpha-fetoprotein (> 400 ng/ml), and vascular invasion are considered major risk factors for extrahepatic disease.23 Identifying patients with increased risk of metastasis can influence the follow-up protocol. In this particularly high-risk population, it is advisable to consider thoracoabdominal scans in order to detect systemic disease early, as the diagnosis of metastatic disease has a major impact on a patient’s management and prognosis.23,24
The treatment goal in early-stage HCC is local tumor eradication, utilizing liver-directed therapies. In this scenario, in patients with low risk of systemic disease, follow-up is focused on the liver, especially after ablation or surgical resection. Also, the option of surgical resection has been explored in patients without portal hypertension and absence of radiological vascular invasion, but with preserved hepatic function, regardless of tumor size.25,26 Because the frequency of micrometastases and vascular invasion rises with tumor size, it is advisable to use a thoracoabdominal radiological follow-up in this uncommon subgroup of patients.
In advanced-stage disease, systemic therapy plays the main role. Beyond treatment with tyrosine kinase inhibitors, immunotherapy is currently gaining acceptance and showing promising results.4 Also, several therapy combinations are actively under investigation, constantly changing the way HCC is treated. In this scenario, thoracoabdominal imaging follow-up is mandatory.
Intermediate-stage HCC represents a very heterogeneous group of disease scenarios, making decisions difficult to generalize. Patients within intermediate-stage HCC are still candidates for local tumor control with LRTs. However, a sustained complete response is not commonly achieved; therefore, the treatment is most often considered palliative. Patients treated with LRT without curative intent may benefit from a follow-up protocol similar to the one followed by those with advanced-stage disease.
Hepatic Tumor Response Assessment
For systemic and local therapies, the only 2 recommended imaging techniques for response assessment are multiphase CT and MRI.1,2 They have comparable diagnostic performance, especially for HCCs larger than 2 cm.27 Below this size cutoff, MRI is considered superior.1,27 There is a trend favoring MRI as the posttreatment imaging of choice, especially after LRTs.28
Locoregional Therapies’ Response Assessment
Tumor ablation and resection are curative-intent therapies employed in early-stage HCC.1,2 The principle of the most popular ablative techniques is tumor destruction, utilizing a thermal or chemical modality.29,30 Ablation techniques that are less widely used include irreversible electroporation and laser ablation.
All of the above-mentioned techniques must achieve complete tumor destruction, including a disease-free margin of at least 0.5 cm to 1 cm.31 Viable tumor is often characterized by areas of arterial enhancement in the ablation/resection margin with venous washout.12
Both CT and MRI can be used to assess the effectiveness of curative-intent therapies. A recurrent or persistent tumor after resection or ablation is often small in size. Because MRI has a better diagnostic performance for small tumor foci detection than CT, it may be the preferred imaging method to evaluate a curative-intent therapy.27 In addition, the use of hepatospecific contrast media improves the diagnostic performance of MRI in this scenario.32
Embolotherapies include a wide variety of techniques that are conventionally considered palliative. They rely on the selective arterial delivery of an embolizing agent, with or without local chemotherapy. Therefore, tumor destruction is mediated by ischemia and the cytotoxic effect of chemotherapy.33
The tumor size and the imaging technique employed for diagnosis are essential factors in considering correct posttreatment follow-up. Transarterial therapies are usually employed in large tumors that are not suitable for ablation/resection. However, in daily practice, small tumors can be treated by embolotherapies when a curative-intent treatment is not technically feasible.34 Conventional TACE (cTACE) is the technique that is most often used and studied. A chemotherapeutic drug is mixed with ethiodized oil resulting in micelles formation. The micelles carry the drug into the tumor and release it selectively over a long time period. This technique ensures a high drug concentration within the tumor, with minimal systemic effect.35
Ethiodized oil contains high amounts of iodine, which makes it intrinsically hyperdense on CT. As such, areas of arterial enhancement could be subtly obscured, limiting the accuracy of an evaluation of response.12 However, the presence of ethiodized oil only minimally affects an MRI signal, so MRI use is advised to evaluate cTACE effectiveness.12 The ethiodized oil-free embolotherapies, such as transarterial embolization and drug-eluting beads–TACE (DEB-TACE), can be evaluated equally with CT or MRI,1,2 as there is no tumoral staining with hyperdense agents.
Care must be taken with the intrinsically radiopaque DEBs that are commercially available (eg LUMI). These particular beads contain iodine within their structure.36 When a high intra- or peritumoral concentration is achieved, a ethiodized oil-like effect can be expected on CT images, so here, too, an MRI assessment may be advisable.
Selective internal radiation therapy (SIRT), also known as TARE, consists of selective intraarterial administration of yttrium-90–coated particles. Its tumoricidal effect relies on direct cell destruction mediated by beta particles that have a penetration range of 2.6 mm to 11 mm.10 The embolic effect of SIRT is minimal, especially when glass spheres are employed, resulting in nonsignificant hypoxia,12 whereas the hallmarks of acute tumor necrosis due to ischemia are often absent after TARE.
TARE is well tolerated by patients with benign or malignant portal thrombosis.37 Arterial embolization in the setting of portal occlusion may lead to severe liver ischemia and necrosis. Also, a TARE variant known as radiation segmentectomy can be performed as a curative-intent treatment for early-stage HCC.5,38
Response assessment after SIRT can be challenging. Radiation-induced tumoral and peritumoral changes are highly heterogeneous and can show different enhancement patterns.12 Tumoral arterial enhancement may not immediately disappear after treatment and may persist for months, even if the tumor was completely treated.39 Therefore, when arterial enhancement persists, progressive tumor shrinkage is the main hallmark of adequate response to TARE.12
Perfusional changes in the radiated liver parenchyma are characterized by arterial enhancement.12 They usually resolve within 6 months but may persist even longer. Therefore, they can be potentially confused with tumoral infiltration. The lack of venous washout and no signal abnormalities on T2 weighted/diffusion images are useful MRI features to distinguish tumoral infiltration from benign post-TARE findings.12
MRI and CT can both be used to assess response. For challenging cases, especially when arterial enhancement persists for longer than expected, MRI may provide more accurate information about tumor viability.12
SBRT uses multiple focused radiation beams to treat HCC. SBRT and SIRT share several posttreatment radiological features, especially the initial minimal effect on arterial enhancement and size.12 In fact, 75% of HCC cases treated with SBRT exhibit persistent arterial enhancement for 3 to 6 months.40,41 Consequently, assessment of SBRT response can be accomplished using the same recommendations as those for SIRT.
No less important is the patient’s compatibility and safety with the selected imaging technique. Patients with an implanted electronic or metallic device should always be checked for compatibility with MRI. Also, metallic artifacts near the liver may degrade image quality, impairing the diagnostic performance of either MRI or CT. The use of contrast media is mandatory for the posttreatment imaging follow-up. Therefore, renal function must always be evaluated before the employment of any gadolinium- or iodine-based contrast media to avoid complications.
Other imaging modalities include contrast-enhanced ultrasound and PET-CT. Both have been extensively studied and are also suggested as valid resources for response assessment.9 However, guidelines currently include only CT and MRI as valid tools in this setting.1,2
Systemic Therapy Response Assessment
For years, the therapeutic armamentarium for advanced-stage HCC was limited to a single drug, sorafenib (Nexavar). Recently, though, new agents, including immunotherapy, have been approved for the treatment of HCC,4 and with this therapeutical evolution comes new challenges in response evaluation.
In contrast to LRTs, in which necrosis is induced by a direct insult focused on the tumor, systemic agents cause tumoral death with multiple mechanisms that create microvasculature changes and immune activation.4 The functional data obtained with CT, MRI, and nuclear medicine can be applied as biomarkers of tumor viability. Tumoral biomarkers, such as metabolism, perfusion, and cellularity, in combination with morphologic changes, may in the future be the new standard of response evaluation.6
Systemic therapy is currently reserved for advanced-stage HCC.42 However, its efficacy in combination with LRT in intermediate-stage disease is being explored in multiple clinical trials.43
Beyond the use of tumor burden in response assessment, a patient’s performance status and liver function are vitally important to consider. Patients with Child-Pugh stage C are unlikely to derive clinical benefit from systemic therapy, and best supportive care should be considered when the patient is not a candidate for liver transplantation.1
Systemic therapy response assessment is not straightforward. It should always be done with multiphase abdominal CT or MRI, plus thoracic imaging. Therefore, CT can be logistically more efficient than MRI. CT, however, can include both the multiphase abdominopelvic exam and a thoracic evaluation. Thoracic MRI is not recommended for oncologic surveillance and abdominal evaluation; it is usually limited to the hepatic area. However, high-quality liver imaging should always be the priority; as such, a CT-MRI combination is feasible and useful.
The multiparametric nature of MRI can accurately depict tumoral necrosis. An MRI “second look” is advisable when CT evaluation is not conclusive. The detection of true necrosis is of main importance, especially when antiangiogenic drugs are used. Changes in the tumoral microvasculature may be represented as the loss of the characteristic arterial enhancement in a viable tumor on CT or MRI.6,44 Rather than a true antitumoral effect, this phenomenon can be explained by a reduction of vascular permeability to contrast agents.44 Despite this, a decrease in arterial enhancement and tumor necrosis are equally accepted indicators of adequate response after systemic therapy.9
Imaging also plays an important role in the surveillance of drug adverse effects, especially when immunotherapy is employed. Bone scintigraphy is not included in the imaging follow-up protocol after systemic therapy.
Imaging Response Criteria
Once timing and imaging technique are established, selecting the imaging response criteria (RC) is the final step in completing the assessment. RC were first created to be used in clinical trials in which tumor response was the primary end point.1,45 Today, RC are commonly employed in daily practice and heavily influence management decisions.46
A multidisciplinary team should make the selection of the RC to be employed. Every health professional involved in the management of HCC must clearly understand how to assess and how to classify response.
Further, imaging RC can be divided into 2 main groups: those based on morphologic tumor burden, and those based on the viability of the tumor burden.6 The main characteristic of tumor viability is arterial enhancement.
Conventional cytotoxic agents induce rapid tumor shrinkage, but adequate response to HCC-specific therapies is not accurately represented by tumor size changes.6,47 Also, posttreatment transient tumoral enlargement is a well-known phenomenon; it should not automatically be considered tumor progression because it could actually be inflammation-mediated.9,45,48
Figure 2 resumes the current and more frequently used response criteria employed for HCC response assessment.
FIGURE 2
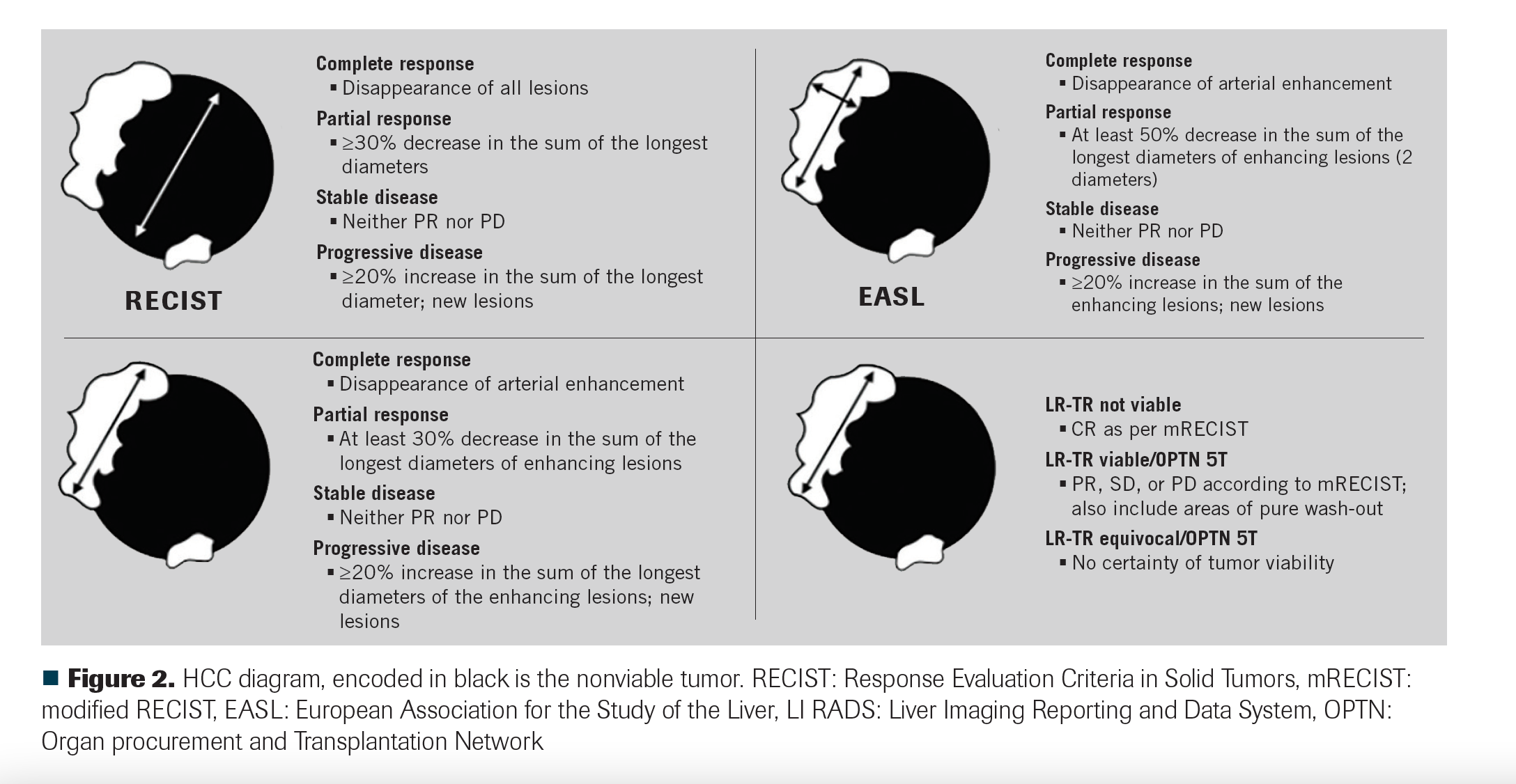
Current viability-based response criteria, such as mRECIST and EASL, were designed specifically for HCC, mainly to assess response after LRTs, although they are also employed for systemic therapy evaluation.49 Metastatic disease is assessed with conventional response criteria, with size and not viability the principal characteristic.45
American College of Radiology Liver Imaging Reporting and Data System (LI-RADS) treatment response criteria (LR-TR) have been introduced into clinical practice and have gained wide acceptance. LI-RADS classifies posttreatment results (LR-TR) into 3 groups: nonviable, viable, and equivocal. However, importantly, LR-TR cannot be used to assess systemic therapy response.18 Unlike mRECIST, the LI-RADS treatment response categories are assigned on a lesion-by-lesion basis and are not assigned to the whole liver or patient.18
An HCC that has been completely treated with a radiation-based modality can retain arterial enhancement, which can be misinterpreted as viable disease by mRECIST and EASL.12 If the LI-RADS response algorithm is employed, a persistent arterial enhancement is best classified as LR-TR equivocal or LR-TR not viable as long as the treated tumor is decreasing in size.12
Inflammation secondary to immunotherapy can cause initial tumoral enlargement. HCC-designed RC may misclassify this phenomena as progression, leading to unnecessary changes or cessation of therapy. iRECIST was developed to assess response on immunotherapy and precisely defines this phenomena as pseudoprogression. It is characterized by an initial increase in the size of lesions, or the visualization of new lesions followed by a response.48
mRECIST is the most studied response system and is frequently compared with RECIST in clinical trials. In several studies, mRECIST has demonstrated an improved prediction of treatment response and overall survival.50-52
The Organ Procurement and Transplantation Network (OPTN) system is employed to allocate patients on the waiting list for liver transplantation in the HCC setting in the United States.53 OPTN class 5 indicates that a liver tumor meets radiological criteria for HCC (analogous to LI-RADS 5). OPTN has a special category to assess response of previously treated HCCs with locoregional therapy. A class OPTN 5T is given to treated HCCs with any residual viable tumor or perfusion defect, corresponding to LI-RADS TR viable or equivocal.54 As in LI-RADS, the OPTN 5T category cannot be applied to assess response to systemic agents.
Conclusions
Response assessment for HCC relies mainly on imaging, and it should take into account 3 essential aspects: timing, imaging technique, and response criteria to be employed.
The first imaging assessment after LR and systemic therapies is usually done after 4 weeks.
After a complete response is achieved, a follow-up schedule based on 3-month intervals is advisable. Mutiphase CT and MRI are the only 2 imaging techniques recommended for HCC response assessment. Contrast-enhanced ultrasound and PET-CT are currently being evaluated for this purpose. MRI diagnostic performance is superior to CT for small tumors (< 2 cm) and those that have been treated with cTACE. HCC that has been treated with radiation-based therapies may show a delayed response, which is characterized by the persistence of arterial enhancement. For accepted radiological response criteria, arterial enhancement is the hallmark of tumor viability. Systemic therapies are continuously evolving; immunotherapies have shown promising results and their response evaluation should include special imaging considerations.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
REFERENCES
1. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. doi:10.1016/j.jhep.2018.03.019.
2. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):871-873. doi:10.1093/annonc/mdy510
3. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
4. Pinter M, Peck-Radosavljevic M. Review article: systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2018;48(6):598-609. doi:10.1111/apt.14913
5. Salem R, Johnson GE, Riaz A, Bishav V, Kim E, Padia S. 992P Yttrium-90 glass microspheres in the treatment of early and advanced hepatocellular carcinoma: the LEGACY study. Ann Oncol. 2020;31(Suppl 4):S692-S693. doi:10.1016/j.annonc.2020.08.1108
6. Hayano K, Fuentes-Orrego JM, Sahani DV. New approaches for precise response evaluation in hepatocellular carcinoma. World J Gastroenterol. 2014;20(12):3059-3068. doi:10.3748/wjg.v20.i12.3059
7. Arora A, Kumar A. Treatment response evaluation and follow-up in hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S126-S129. doi:10.1016/j.jceh.2014.05.005
8. Crocetti L, Della Pina C, Cioni D, Lencioni R. Peri-intraprocedural imaging: US, CT, and MRI. Abdom Imaging. 2011;36(6):648-660. doi:10.1007/s00261-011-9750-9
9. Yaghmai V, Besa C, Kim E, Gatlin JL, Siddiqui NA, Taouli B. Imaging assessment of hepatocellular carcinoma response to locoregional and systemic therapy. AJR Am J Roentgenol. 2013;201(1):80-96. doi:10.2214/AJR.13.10706
10. Ibrahim SM, Nikolaidis P, Miller FH, et al. Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom Imaging. 2009;34(5):566-581. doi:10.1007/s00261-008-9454-y
11. Singh P, Anil G. Yttrium-90 radioembolization of liver tumors: what do the images tell us?. Cancer Imaging. 2014;13(4):645-657. doi:10.1102/1470-7330.2013.0057
12. Mendiratta-Lala M, Masch WR, Shampain K, et al. MRI assessment of hepatocellular carcinoma after local-regional therapy: a comprehensive review. Radiology: Imaging Cancer. 2020;2(1):e190024. doi:10.1148/rycan.2020190024
13. Boonsirikamchai P, Loyer EM, Choi H, Charnsangavej C. Planning and follow-up after ablation of hepatic tumors: imaging evaluation. Surg Oncol Clin N Am. 2011;20(2):301-315, viii. doi:10.1016/j.soc.2010.11.007
14. Lim HK, Choi D, Lee WJ, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221(2):447-454. doi:10.1148/radiol.2212010446
15. Crocetti L, Lencioni R. Thermal ablation of hepatocellular carcinoma. Cancer Imaging. 2008;8(1):19-26. doi:10.1102/1470-7330.2008.0004
16. Bouda D, Lagadec M, Alba CG, et al. Imaging review of hepatocellular carcinoma after thermal ablation: the good, the bad, and the ugly. J Magn Reson Imaging. 2016;44(5):1070-1090. doi:10.1002/jmri.25369
17. Bruix J, Sherman M, Llovet JM, et al; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421-430. doi:10.1016/s0168-8278(01)00130-1
18. Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289(3):816-830. doi:10.1148/radiol.2018181494
19. Bajpai S, Kambadakone A, Guimaraes AR, Arellano RS, Gervais DA, Sahani D. Image-guided treatment in the hepatobiliary system: role of imaging in treatment planning and posttreatment evaluation. Radiographics. 2015;35(5):1393-1418. doi:10.1148/rg.2015140281
20. Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33(1):11-17. doi:10.1007/s00270-009-9736-y
21. Koneru B, Teperman LW, Manzarbeitia C, et al. A multicenter evaluation of utility of chest computed tomography and bone scans in liver transplant candidates with stages I and II hepatoma. Ann Surg. 2005;241(4):622-628. doi:10.1097/01.sla.0000157267.27356.80
22. Kutaiba N, Ardalan Z, Patwala K, Lau E, Goodwin M, Gow P. Value of bone scans in work-up of patients with hepatocellular carcinoma for liver transplant. Transplant Direct. 2018;4(12):e408. doi:10.1097/TXD.0000000000000846
23. Yokoo T, Patel AD, Lev-Cohain N, Singal AG, Yopp AC, Pedrosa I. Extrahepatic metastasis risk of hepatocellular carcinoma based on α-fetoprotein and tumor staging parameters at cross-sectional imaging. Cancer Manag Res. 2017;9:503-511. doi:10.2147/CMAR.S147097
24. Sala M, Forner A, Varela M, Bruix J. Prognostic prediction in patients with hepatocellular carcinoma. Semin Liver Dis. 2005;25(2):171-180. doi:10.1055/s-2005-871197
25. Zamora-Valdes D, Taner T, Nagorney DM. Surgical treatment of hepatocellular carcinoma. Cancer Control. 2017;24(3):1073274817729258. doi:10.1177/1073274817729258
26. Lim C, Mise Y, Sakamoto Y, et al. Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World J Surg. 2014;38(11):2910-2918. doi:10.1007/s00268-014-2704-y
27. Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging – a systematic review and meta-analysis. Radiology. 2015;275(1):97-109. doi:10.1148/radiol.14140690
28. Kloeckner R, Otto G, Biesterfeld S, Oberholzer K, Dueber C, Pitton MB. MDCT versus MRI assessment of tumor response after transarterial chemoembolization for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33(3):532-540. doi:10.1007/s00270-009-9728-y
29. Lencioni R, Crocetti L, Della Pina MC, Cioni D. Percutaneous image-guided radiofrequency ablation of liver tumors. Abdom Imaging. 2009;34(5):547-556. doi:10.1007/s00261-008-9479-2
30. Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72(Suppl 1):124-131. doi:10.1159/000111718
31. Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88(11):2452-2463.
32. Granata V, Petrillo M, Fusco R, et al. Surveillance of HCC patients after liver RFA: role of MRI with hepatospecific contrast versus three-phase CT scan – experience of high volume oncologic institute. Gastroenterol Res Pract. 2013;2013:469097. doi:10.1155/2013/469097
33. Huppert P. Current concepts in transarterial chemoembolization of hepatocellular carcinoma. Abdom Imaging. 2011;36(6):677-683. doi:10.1007/s00261-011-9755-4
34. Reig M, Darnell A, Forner A, Rimola J, Ayuso C, Bruix J. Systemic therapy for hepatocellular carcinoma: the issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Semin Liver Dis. 2014;34(4):444-455. doi:10.1055/s-0034-1394143
35. Meza-Junco J, Montano-Loza AJ, Liu DM, et al. Locoregional radiological treatment for hepatocellular carcinoma: which, when and how? Cancer Treat Rev. 2012;38(1):54-62. doi:10.1016/j.ctrv.2011.05.002
36. Reicher J, Mafeld S, Priona G, et al. Early experience of trans-arterial chemo-embolisation for hepatocellular carcinoma with a novel radiopaque bead. Cardiovasc Intervent Radiol. 2019;42(11):1563-1570. doi:10.1007/s00270-019-02317-3
37. Lau W-Y, Sangro B, Chen P-J, et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology. 2013;84(5):311-318. doi:10.1159/000348325
38. Lewandowski RJ, Gabr A, Abouchaleh N, et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287(3):1050-1058. doi:10.1148/radiol.2018171768
39. Adcock CS, Florez E, Zand KA, Patel A, Howard CM, Fatemi A. Assessment of treatment response following yttrium-90 transarterial radioembolization of liver malignancies. Cureus. 2018;10(6):e2895. doi:10.7759/cureus.2895
40. Mendiratta-Lala M, Masch W, Shankar PR, et al. Magnetic resonance imaging evaluation of hepatocellular carcinoma treated with stereotactic body radiation therapy: long term imaging follow-up. Int J Radiat Oncol Biol Phys. 2019;103(1):169-179. doi:10.1016/j.ijrobp.2018.09.004
41. Mendiratta-Lala M, Gu E, Owen D, et al. Imaging findings within the first 12 months of hepatocellular carcinoma treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102(4):1063-1069. doi:10.1016/j.ijrobp.2017.08.022
42. Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48(4):1312-1327. doi:10.1002/hep.22506\
43. Xu W, Liu K, Chen M, et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019;11:1758835919862692. doi:10.1177/1758835919862692
44. Taylor M, Rössler J, Geoerger B, Vassal G, Farace F. New anti-angiogenic strategies in pediatric solid malignancies: agents and biomarkers of a near future. Expert Opin Investig Drugs. 2010;19(7):859-874. doi:10.1517/13543784.2010.487654
45. Henze J, Maintz D, Persigehl T. RECIST 1.1, irRECIST 1.1, and mRECIST: how to do. Curr Radiol Rep. 2016;4:48. doi:10.1007/s40134-016-0178-4
46. El-Maraghi RH, Eisenhauer EA. Review of phase II trial designs used in studies of molecular targeted agents: outcomes and predictors of success in phase III. J Clin Oncol. 2008;26(8):1346-1354. doi:10.1200/JCO.2007.13.5913
47. Mannelli L, Kim S, Hajdu CH, Babb JS, Clark TWI, Taouli B. Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. AJR Am J Roentgenol. 2009;193(4):1044-1052. doi:10.2214/AJR.08.1461
48. Seymour L, Bogaerts J, Perrone A, et al; RECIST Working Group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
49. Edeline J, Boucher E, Rolland Y, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118(1):147-156. doi:10.1002/cncr.26255
50. Hyun D, Shin SW, Cho SK, et al. Efficacy of RECIST and mRECIST criteria as prognostic factors in patients undergoing repeated iodized oil chemoembolization of intermediate stage hepatocellular carcinoma. Acta Radiol. 2015;56(12):1437-1445. doi:10.1177/0284185114560937
51. Sato Y, Watanabe H, Sone M, et al; Japan Interventional Radiology in Oncology Study Group – JIVROSG. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013;118(1):16-22. doi:10.3109/03009734.2012.729104
52. Shim JH, Lee HC, Kim S-O, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? a validation study of old and new models. Radiology. 2012;262(2):708-718. doi:10.1148/radiol.11110282
53. Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266(2):376-382. doi:10.1148/radiol.12121698
54. Elsayes KM, Kielar AZ, Agrons MM, et al. Liver Imaging Reporting and Data System: an expert consensus statement. J Hepatocell Carcinoma. 2017;4:29-39. doi:10.2147/JHC.S125396
