Non-Secretory Myeloma: A Clinician’s Guide
Numerous small series of patients suggest that the prognosis for non-secretory myeloma patients is likely no worse than the prognosis for patients with traditional secretory myeloma, and in some settings may be superior.
The treatment of patients with multiple myeloma has dramatically changed over the past 10 years due to an improved understanding of plasma cell biology and the development of new targets. The subset of these patients with non-secretory myeloma-a group of patients who do not secrete immunoglobulin or its component parts into either the blood or urine-has been challenging to treat and to assess for disease response. Newer methods of assessment for plasma cell disorders, such as the widely used serum free light chain assay, have reduced the number of patients with truly non-secretory myeloma to less than 3% of all newly diagnosed myeloma patients. With regard to prognosis, it appears from most series that patients with non-secretory myeloma have a prognosis similar to or better than that of patients with secretory myeloma. This has not been evaluated in populations from which patients with free light–only disease are excluded, but there is no reason to expect that outcomes in patients with non-secretory myeloma will be appreciably worse, since many harbor the t(11;14) translocation. Finally, imaging with positron emission tomography (PET)/CT scans, and minimal residual disease (MRD) assessment with multi-parameter flow cytometry, may provide newer methods for response assessment, something that has been severely limited in these patients due to the lack of a reliable biomarker. Future directions in response assessment include the amalgamation of imaging and MRD assessment, which may enhance our ability to assess response both in patients with non-secretory myeloma and in other patients with myeloma.
Introduction
Multiple myeloma is a disorder characterized by the presence of clonal plasma cells in the marrow, which results in end-organ damage, as manifested by hematologic, renal, or bone complications.[1] Myeloma may be preceded by a premalignant phase in which clonal plasma cells are present but there is no evidence of end-organ damage (this is known as “monoclonal gammopathy of unknown significance” [MGUS] or “smoldering myeloma”).[2] However, a hallmark of most cases of multiple myeloma and its antecedent phases is the persistent production of some form of immunoglobulin, either a complete antibody (heavy and light chain) or the individual components of monoclonal antibodies (heavy chain or light chain). It is often this protein production that calls the disease to attention in the smoldering or MGUS stage, since patients frequently have no other signs of disease.[3]
A unique feature of malignant plasma cells is continued protein production, a function that is typically lost when cells are transformed from a normal to a malignant phenotype. In addition, the protein produced can often serve as a reliable biomarker for disease presence. The availability of this protein in the blood or urine for quantitative assessment using serum protein electrophoresis (SPEP), urine protein electrophoresis (UPEP), or the serum free light chain assay facilitates disease response assessment in most cases of myeloma, since the assessment can be done with routine blood and urine testing rather than requiring imaging or bone marrow assessment, as in other hematologic malignancies.[4] While the concept of biomarker assessment as a surrogate for response is useful in most cases of myeloma, in patients with high-risk disease, light chain or non-secretory escape may occur, likely as a consequence of clonal evolution: although a protein was initially produced, it is lost with relapse. This possibility, in conjunction with the fact that relapse remains inevitable even in patients who achieve a complete remission, as determined via serum and urine studies, has led a number of groups to evaluate more stringent methods for assessing disease status,[5-9] and it is likely that these methods will prove useful as we seek to define the optimal methods for assessing response and disease status in patients with non-secretory myeloma.
Biology of Non-Secretory Myeloma
Myeloma is characterized by the clonal infiltration of the marrow by plasma cells that typically produce a serum or urine paraprotein. The serum protein is often characterized by an intact immunoglobulin (heavy and light chain), or it may be characterized by the light chain alone. In the urine, an intact immunoglobulin is also often present, although some patients have predominantly light chain in the urine (typically referred to as “Bence-Jones protein”).
Years ago it was determined that in some patients with non-secretory myeloma, immunohistochemical staining of the marrow plasma cells demonstrated the presence of immunoglobulin molecules, while in others there was no evidence of immunoglobulin production by the plasma cells.[10-12] This observation allows us to divide non-secretory myeloma patients into several groups. The first group consists of patients who are “non-producers.” These are patients whose tumors may have defects in immunoglobulin synthesis. While these tumors may have all the features of a plasma cell disorder, they are not able to synthesize or secrete a protein.[13] Patients who have no measurable protein in the blood or urine, yet who still have a significant plasma cell burden in the marrow and evidence of end-organ damage, fall into this category. In these patients, even use of the free light chain assay will not reveal measurable disease as currently defined, since they do not make a protein.
The next category of non-secretory myeloma patients consists of those whose tumors produce a protein but have defects in secretion. It has been demonstrated in vitro that a single amino acid substitution in a light chain can potentially block secretion outside of the cell, and in a patient sample, it has been demonstrated that a mutation in the immunoglobulin gene can account for a lack of secretion in a patient with non-secretory myeloma.[14] However, among those patients whose tumors have defects in Ig secretion, there is a subset of patients who have impaired secretion but are able to secrete some low levels of light chains. These are patients who have oligosecretory myeloma, and while their protein secretion may not be as high as that seen in typical myeloma, they are able to secrete some proteins and these can be measured using current technology.
Patients who may initially be categorized as non-secretory, who have a protein that cannot be detected by either SPEP or UPEP (both with immunofixation), often fall into a more recently identified group who have “free light–only” myeloma. These are patients in whom disease can be revealed only with the free light assay, which is known to more accurately detect kappa and lambda light chains in the blood.[15,16] Drayson et al noted that in a series of 28 patients, 19 had elevations in the serum kappa or lambda light chains, as measured by the free light assay.[17] It is important to note that while serum measurement of free light chains has now become a standard diagnostic test for plasma cell disorders,[18] disease assessment with the urinary free light chain assay is notoriously unreliable, and this approach should not be routinely used in clinical practice. The urinary free light chain assay is different from the UPEP with immunofixation, which remains the optimal study to use when assessing urinary protein production.
TABLE 1
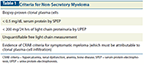
Criteria for Non-Secretory Myeloma
Those patients in whom more modern immunoglobulin testing methods can detect no serum, urine, or free light–based disease are the patients with truly non-secretory myeloma (Table 1).
The absolute frequency of non-secretory myeloma can be difficult to analyze, and such quantification is, in fact, even more challenging as we enter an era of different methods of response assessment. In the era before the routine use of the free light assay, Kyle et al estimated the frequency of non-secretory myeloma to be around 3% in a cohort of 1,027 patients with newly diagnosed myeloma.[19] When these non-secretory patients were followed over time, 22 of the 29 patients initially deemed non-secretory remained non-secretory over the course of their disease. When more modern immunoglobulin testing methods are used, the patients in the non-secretory category still represent around 3% of all myeloma patients at the time of diagnosis; however, as patients live longer, it has been noted that, through clonal evolution, others may develop non-secretory myeloma as a consequence of long-term treatment (“light chain escape phenomenon”). The loss of protein production with subsequent disease progression that these increasing numbers reflect is typically seen in patients with high-risk myeloma and is often associated with a “de-differentiated” pathological specimen that may or may not express CD138 or CD38, and that often expresses CD20.
As mentioned earlier, non-secretory myeloma must also be distinguished from oligosecretory myeloma, in which proteins are produced but at very low levels that make reliable measurement more challenging. Oligosecretory multiple myeloma is often characterized by serum protein of < 1.0 g/dL, urine protein of < 200 mg/24 hrs, and free light chain values of < 100 mg/L (or 10 mg/dL).[15]
Clinically, patients who present with true non-secretory disease at diagnosis behave differently from patients who present with oligosecretory disease at the outset, as well as from those who progress from having secretory disease at diagnosis to oligosecretory or non-secretory disease at the time of relapse. These latter patients typically have high-risk myeloma, genomic instability, and rapid clonal evolution.
Diagnosis and Workup of Non-Secretory Patients
TABLE 2
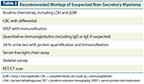
Recommended Workup of Suspected Non-Secretory Myeloma
Given the paucity of information on these patients, how should the clinician approach the workup and treatment of patients with known or suspected non-secretory myeloma? The standard workup for newly diagnosed myeloma as recommended by the consensus statement from the International Myeloma Workshop[2] is a good place to start. According to this statement, the workup for all newly diagnosed myeloma patients includes SPEP, UPEP, and serum free light chain assay, in addition to basic imaging using a skeletal survey (Table 2). Patients with light chain myeloma may have only a serum free light chain abnormality; in modern parlance, these patients are not considered to have true non-secretory myeloma. Although in older series these patients were reported to have non-secretory disease, the free light chain assay now provides us with a biomarker that can be used to assess response. However, there remains a group who comprise around 2% to 4% of patients and for whom there is no measurable biomarker and thus no serum, urine, or free light chain–based method of response assessment. These patients represent the subset with true non-secretory myeloma. In these patients, positron emission tomography (PET)/CT imaging can serve as a relatively objective assay that can be used, along with marrow plasmacytosis, to assess the level of disease response; PET/CT imaging can help identify sites of bone disease, and after therapy, it can be used to evaluate active vs quiescent bone lesions in the context of therapy.
Prognosis
The characteristics of patients with non-secretory myeloma have been reported in a number of small series. In a series from France, it was reported that there was a higher proportion of patients with the t(11;14) translocation among patients with non-secretory myeloma, as well as among those with IgM and IgE myelomas. There was no clear biologic reason for this unique feature; nonetheless, the frequency of this translocation in non-secretory myeloma patients was 83% in a cohort of 24 patients. This may in part account for differences in outcomes for non-secretory myeloma patients.
A few series have been reported that compared the outcomes of non-secretory patients with those of more traditional secretory myeloma patients. In a group of 127 myeloma patients from the UK who had undergone transplantation, 6 were found to be patients with non-secretory disease; the overall survival (OS) and progression-free survival (PFS) in that small group of patients were found to be superior to those of the patients with a more traditional secretory myeloma phenotype.[20] The PFS for this group was 36 months following high-dose therapy (HDT), while the PFS for all the other secretory groups in aggregate was 23 months. The authors of this paper suggest that enhanced sensitivity to HDT may be a feature of a less mature plasma cell phenotype that is not secreting immunoglobulin; however, an alternative hypothesis could be that there is a lower frequency of high-risk genetic alterations in the non-secretory patients, which allows for their improved outcomes compared with the outcomes of patients with IgG, IgA, or light chain myelomas.[21] It has been suggested by others that patients with light chain or IgA myeloma may harbor a higher frequency of the t(4;14) translocation or other high-risk features that account for their shorter PFS and OS.
In the series from Kyle et al, the outcomes for patients with non-secretory myeloma were similar to those of patients with secretory myeloma, with a median OS of 38 months for the non-secretory patients vs 33.4 months for the remaining patients in the 1,027-patient cohort.[19]
Finally, in a series from the Center for International Blood & Marrow Transplant Research (CIBMTR), which assembled the largest set of non-secretory patients to date, 110 patients with non-secretory myeloma were compared with matched controls in a 4:1 fashion. This study showed no difference in PFS or OS between the non-secretory patients and the matched controls.[22] All patients in this series underwent HDT and autologous transplant, but the data on how many of these patients had free light–measurable disease (true non-secretors vs those with oligosecretory disease) was not available. There also was insufficient information on genetics and fluorescence in situ hybridization (FISH) results to determine whether the molecular basis for either similar or improved outcomes for non-secretory patients lies in the underlying biology of the disease-although the authors do speculate that this is likely the cause.
Treatment of Non-Secretory Myeloma
The management of newly diagnosed myeloma has changed dramatically now that we have new agents to use in all phases of treatment. Nowhere is the explosion in new regimens more apparent than in the setting of induction therapy. Three-drug combinations, in which proteasome inhibitors are combined with immunomodulatory agents or alkylating agents, are commonly used induction regimens for patients being considered for HDT and autologous transplant.[1] With improved inductions, a greater proportion of patients are achieving a complete response, and through novel consolidation and maintenance therapy, patients are now achieving molecular or flow cytometric complete remission as well.[23]
While there are no studies of large numbers of non-secretory myeloma patients, an analysis by Nooka et al at our center evaluated the PFS and OS for patients receiving lenalidomide, bortezomib, and dexamethasone (RVD) induction followed by either early or late transplant.[24] Among all patients, the 3-year OS was > 85%, and this appeared to be similar in all analyzed patients, and in the small subset of patients in the same cohort who were defined as non-secretory. These results-in conjunction with those from the larger series of patients reported earlier in this paper,[19] which provide evidence that as the OS for patients with myeloma has improved, the OS for non-secretory patients has improved by a similar magnitude-suggest that the gains in outcomes associated with the use of new agents are similar for secretory and non-secretory myeloma. Thus, optimal induction likely includes the use of a three-drug combination that involves either proteasome inhibitors together with immunomodulatory agents (RVD[25] or bortezomib, thalidomide, and dexamethasone [VTD][26,27]), or proteasome inhibitors in combination with alkylating agents (bortezomib, cyclophosphamide, and dexamethasone [VCD]). Patients not considered to be suitable for triple combinations may be treated with doublets, such as lenalidomide/dexamethasone (RD)[28] or bortezomib/dexamethasone (VD).[29]
Disease Assessment and Response
Response assessment in myeloma is typically based on clearance of measurable protein, with the latest definition of complete response requiring no detectable protein in the blood or urine and a normal free light ratio. Even with a response that meets these criteria, more in-depth assessment using molecular techniques, such as polymerase chain reaction (PCR) testing,[30,31] DNA sequencing, and multi-parameter flow cytometry (MPF),[6,7,32] is able to identify remaining disease burden that likely will contribute to relapse.
Non-secretory myeloma typically presents with cytopenias or bone disease; thus, objective measures of disease response include marrow assessment and imaging studies.[16] While marrow involvement in the context of most myelomas can be patchy and inconsistent, in the setting of non-secretory disease, marrow involvement may be the only true objective measure of disease burden.
It should be noted that while marrows have historically been used as the sole measure of disease response and activity in the setting of non-secretory myeloma, assessment of disease burden using routine marrow histology and routine flow cytometry is notoriously inaccurate, due in large part to the patchy nature of marrow involvement and issues with sampling.[6] However, the use of specialized flow cytometry, as championed by the Spanish Myeloma Group, has allowed us to more intensely evaluate the marrow, thus making marrow evaluation a more reliable means of disease assessment. Rather than using flow cytometry as a method for assessing crude disease involvement, MPF is now being used as a measure of minimal residual disease (MRD), and MPF results have not only predictive but also prognostic implications in the setting of disease assessment post-transplant.[7]
TABLE 3

Recommended Tests to Assess Response and Disease Status in a Patient With Non-Secretory Myeloma
However, while assessment of disease using MPF is a significant improvement over conventional flow cytometry or histologic assessment of plasma cell number, MPF alone is probably not sufficient to assess total body myeloma burden. For this reason, the pairing of imaging and more sensitive marrow assessment represents an optimal method by which to assess response to therapy and MRD, and will likely be applied to all myeloma patients, not just non-secretory patients in whom the inability to use SPEP or UPEP limits methods of response assessment (Table 3).
For serial assessment of bone disease, a skeletal survey is inadequate for identifying active vs quiescent bone lesions; however, PET imaging is potentially able to help bridge the gap in this instance.[8,9] Patients with non-secretory or oligosecretory myeloma often not only have bone-based lytic disease, but may also have extra-medullary manifestations of disease, which are best evaluated using serial PET/CT scans. Quiescent bone disease may show up as lytic bone disease on a CT scan but is typically not fluorodeoxyglucose (FDG)-avid (a feature that would allow the clinician to more fully assess the efficacy of therapy). It should be noted that marrow and imaging assessment together is the best method for assessing response in non-secretory disease, since the two technologies often complement one another. It is likely that, in the near future, MRD assessment and imaging with more modern methods, such as PET/CT, will become more routine in secretory myeloma as well.
Conclusion
The diagnosis of non-secretory myeloma has evolved as our methods for assessing protein and now plasma cell numbers in the marrow have increased in sensitivity. While this development has reduced the number of patients defined as having non-secretory myeloma, it has not changed the challenge we clinicians have in determining response, and depth or duration of response. Fortunately, numerous small series of patients suggest that the prognosis for these patients is likely no worse than the prognosis for patients with traditional secretory myeloma, and in some settings may be superior. The use of MPF in conjunction with PET/CT scanning provides the opportunity to more formally assess low levels of disease regardless of the secretory potential of the malignant plasma cell, and this combination approach will likely be used to determine response and possibly cure in all patients in the near future. A careful and thorough initial evaluation is key to arriving at the correct data, which are necessary in order to best define a long-term strategy for disease control and assessment.
Financial Disclosure:Dr. Lonial serves as a consultant for Bristol-Myers Squibb, Celgene, Janssen, Millennium, Novartis, and Onyx. Dr. Kaufman serves as a consultant for Celgene, Janssen, Millennium, Novartis, and Onyx; he receives research support from Celgene, Merck, and Novartis.
References:
REFERENCES
1. Lonial S, Miguel JF. Induction therapy for newly diagnosed multiple myeloma. J Natl Compr Canc Netw. 2013;11:19-28.
2. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701-5.
3. Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860-73.
4. Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691-5.
5. Paiva B, Gutierrez NC, Rosinol L, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119:687-91.
6. Paiva B, Martinez-Lopez J, Vidriales MB, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29:1627-33.
7. Paiva B, Vidriales MB, Cervero J, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112:4017-23.
8. Zamagni E, Cavo M. The role of imaging techniques in the management of multiple myeloma. Br J Haematol. 2012;159:499-513.
9. Zamagni E, Patriarca F, Nanni C, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989-95.
10. Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am. 1999;13:1259-72.
11. Smith DB, Harris M, Gowland E, et al. Non-secretory multiple myeloma: a report of 13 cases with a review of the literature. Hematol Oncol. 1986;4:307-13.
12. Ma ES, Shek TW, Ma SY. Non-secretory plasma cell myeloma of the true non-producer type. Br J Haematol. 2007;138:561.
13. Decourt C, Galea HR, Sirac C, Cogne M. Immunologic basis for the rare occurrence of true nonsecretory plasma cell dyscrasias. J Leukoc Biol. 2004;76:528-36.
14. Coriu D, Weaver K, Schell M, et al. A molecular basis for nonsecretory myeloma. Blood. 2004;104:829-31.
15. Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:2220.
16. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749-57.
17. Drayson M, Tang LX, Drew R, et al. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001;97:2900-2.
18. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215-24.
19. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33.
20. Terpos E, Apperley JF, Samson D, et al. Autologous stem cell transplantation in multiple myeloma: improved survival in nonsecretory multiple myeloma but lack of influence of age, status at transplant, previous treatment and conditioning regimen. A single-centre experience in 127 patients. Bone Marrow Transplant. 2003;31:163-70.
21. Avet-Loiseau H, Garand R, Lode L, et al. Translocation t(11;14)(q13;q32) is the hallmark of IgM, IgE, and nonsecretory multiple myeloma variants. Blood. 2003;101:1570-1.
22. Kumar S, Perez WS, Zhang MJ, et al. Comparable outcomes in nonsecretory and secretory multiple myeloma after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:1134-40.
23. Cavo M, Rajkumar SV, Palumbo A, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117:6063-73.
24. Nooka A, Langston A, Waller EK, et al. Early versus delayed autologous stem cell transplant (ASCT)in patients receiving induction therapy with lenalidomide, bortezomib, and dexamethasone (RVD) for newly diagnosed multiple myeloma (MM). Presented at ASCO 2013 Annual Meeting; May 31 – Jun 4, 2013; Chicago, IL; Abstract 8540.
25. Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679-86.26. Kaufman JL, Nooka A, Vrana M, et al. Bortezomib, thalidomide, and dexamethasone as induction therapy for patients with symptomatic multiple myeloma: a retrospective study. Cancer. 2010;116:3143-51.
27. Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075-85.
28. Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29-37.
29. Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621-9.
30. Corradini P, Cavo M, Lokhorst H, et al. Molecular remission after myeloablative allogeneic stem cell transplantation predicts a better relapse-free survival in patients with multiple myeloma. Blood. 2003;102:1927-9.
31. Ladetto M, Pagliano G, Avonto I, et al. Consolidation with bortezomib, thalidomide and dexamethasone induces molecular remissions in autografted multiple myeloma patients. ASH Annual Meeting Abstracts. 2007;110:530.
32. Paiva B, Almeida J, Perez-Andres M, et al. Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry B Clin Cytom. 2010;78:239-52.