Nonmyeloablative Preparative Regimens for Allogeneic Hematopoietic Transplantation
High-dose myeloablative therapy with allogeneic hematopoietictransplantation is an effective treatment for hematologic malignancies,but this approach is associated with a high risk of complications.The use of relatively nontoxic, nonmyeloablative, or reduced-intensitypreparative regimens still allows engraftment and the generation ofgraft-vs-malignancy effects, is potentially curative for susceptiblemalignancies, and reduces the risk of treatment-related morbidity.Two general strategies along these lines have emerged, based on theuse of (1) immunosuppressive chemotherapeutic drugs, usually apurine analog in combination with an alkylating agent, and (2) lowdosetotal body irradiation, alone or in combination with fludarabine(Fludara).
ABSTRACT: High-dose myeloablative therapy with allogeneic hematopoietic transplantation is an effective treatment for hematologic malignancies, but this approach is associated with a high risk of complications. The use of relatively nontoxic, nonmyeloablative, or reduced-intensity preparative regimens still allows engraftment and the generation of graft-vs-malignancy effects, is potentially curative for susceptible malignancies, and reduces the risk of treatment-related morbidity. Two general strategies along these lines have emerged, based on the use of (1) immunosuppressive chemotherapeutic drugs, usually a purine analog in combination with an alkylating agent, and (2) lowdose total body irradiation, alone or in combination with fludarabine (Fludara).
High-dose myeloablative therapy with allogeneic hematopoietic transplantation is an effective yet risky treatment for hematologic malignancies. This strategy is associated with a high risk of treatment-related complications, ranging from 10% to > 50%, depend depending on histocompatibility, age comorbidities, and disease factors. The initial concept of allogeneic transplantation for treatment of cancer was to use the transplant for supportive care, as a means to restore hematopoiesis after myeloablative doses of chemotherapy and/or total body irradiation.[1] The pretransplant "preparative regimen" was intended to eradicate the malignancy as well as provide sufficient immunosuppression to prevent graft rejection.
The therapeutic benefit of allogeneic marrow transplantation for many diagnoses is largely related to an associated immune-mediated graftvs- malignancy effect.[2,3] This realization has recently led to the use of less toxic, nonmyeloablative preparative regimens to achieve engraftment and allow development of graft-vs-malignancy effects as a primary form of therapy.[4] For nonmalignant disorders, it is not necessary to ablate diseased tissues; it is only necessary to achieve mixed chimerism to provide a source of normal hematopoietic cells.
Two general strategies have emerged. One approach is based on the use of immunosuppressive chemotherapeutic drugs, usually a purine analog in combination with an alkylating agent.[5-7] The other technique is based on the immunosup pressive effects of low-dose total body irradiation, alone or in combination with fludarabine (Fludara).[8]
Nonmyelablative vs Reduced-Intensity Regimens
FIGURE 1

Commonly Used Nonablative or Reduced-Toxicity Regimens
Several regimens have been proposed to reduce the toxicity associated with allogeneic transplantation. The relative myelosuppressive and immunosuppressive effects of these regimens are summarized in Figure 1.
As a working definition, a truly nonmyeloablative regimen should not eradicate host hematopoiesis and should allow relatively prompt hematopoietic recovery (< 28 days) without a transplant.[4] Upon engraftment, mixed chimerism should be present, with both donor- and recipient- derived cells detectable. If the graft is rejected, prompt autologous recovery should occur. This category includes the fludarabine/cyclophosphamide (Cytoxan, Neosar),[6] fludarabine/idarubicin (Idamycin)/ cytarabine,[9] and 2-Gy total body irradiation-based regimens.[8]
FIGURE 2
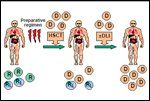
Nonablative Hematopoietic Transplantation for Hematologic Malignancy
The general treatment scheme of a nonablative regimen is illustrated in Figure 2. The nonablative preparative regimen does not completely eliminate host normal and malignant cells. An allogeneic graft-vs-hematopoietic effect occurs in which donor cells eradicate residual host hematopoiesis. Graft-vs-malignancy effects generally occur after the development of full donor T-cell chimerism.[10]
An ablative regimen requires hematopoietic transplantation for recovery, and only donor-derived cells should be detectable after engraftment. Many of the lower-dose regimens designed to reduce toxicity- including combinations of intermediate doses of melphalan (Alkeran) or busulfan (Busulfex, Myleran) with fludarabine[5,7]-have been referred to as nonmyeloablative, but do not actually meet nonmyeloablative criteria. These regimens require a transplant for hematologic recovery, and if the graft is rejected, prolonged aplasia typically occurs. They should be referred to as reduced-intensity ablative regimens.
The intensity of immunosuppression required for engraftment depends on the immunocompetence of the recipient, histocompatibility, and the composition of the transplant. Initial studies focused on patients with a human leukocyte antigen (HLA)- matched sibling donor. More intensive regimens are required for engraftment in settings of greater genetic disparity, including unrelated-donor or HLAnonidentical transplants.
Advantages of Nonablative Regimens
Nonablative regimens have been studied as a means to reduce regimen- related toxicity. This approach allows hematopoietic transplantation in patients considered ineligible for myeloablative preparative regimens because of advanced age or the presence of comorbidities. Nonablative transplants are also associated with a reduced incidence and severity of acute graft-vs-host disease (GVHD).[11] Several factors likely contribute to this observation. The clinical manifestations of acute GVHD partly result from the toxicity of the preparative regimen and subsequent cytokine release as well as the alloreactivity of the graft.[12] Residual host T cells may produce a "veto" effect that also inhibits development of GVHD, and GVHD is less severe in the setting of mixed chimerism.
Infectious complications also appear to be reduced. Neutropenia is reduced or eliminated by most nonablative regimens.[13] In addition, since the nonablative preparative regimen does not immediately eliminate host-derived immunocompetent cells, these cells can contribute to host defense in the early posttransplant period.
Disadvantages of Nonablative Transplants
There are also potential disadvantages of using nonablative preparative regimens. Higher doses of busulfan or total body irradiation have been shown to reduce the risk of relapse in chronic myelogenous leukemia (CML) and acute myelogenous leukemia (AML).[14-16] Approximately one-third of patients with good risk leukemias are cured with high-dose therapy and syngeneic transplants in which graft-vsleukemia (GVL) effects would not be expected to occur.[17] Young patients without comorbidities tolerate supralethal regimens well, and reducing toxicity may not improve their survival. Given these considerations, nonablative regimens should be reserved for diagnoses that are exquisitely sensitive to graft-vs-malignancy effects, for older patients, or for those with comorbidities who would not be able to tolerate an ablative regimen.
Disease Susceptibility to Graft-vs-Malignancy Effects
TABLE 1

Disease Sensitivity to Graft-vs-Malignancy Effects
Malignancies differ greatly in their susceptibility to GVL effects and, hence, their sensitivity to nonmyeloablative allogeneic transplants (see Table 1). Three general categories,based on levels of sensitivity to GVL effects, can be defined.
• Highly Sensitive Malignancies-CML is the disease in which GVL effects have been best documented.[ 18] The majority of CML patients who relapse into chronic phase following an allogeneic transplant achieve a durable complete remission with donor lymphocyte infusions.[19] Indolent lymphoid malignancies also appear to be very sensitive to graft-vs-malignancy effects, as evidenced by durable remissions to donor lymphocyte infusions or modulation of immunosuppression in patients who have relapsed after an allogeneic transplant.[20] Allogeneic transplants are associated with a substantially lower relapse rate than purged autologous transplants.
Selected patients with chronic lymphocytic leukemia or low-grade lymphoma have responded to donor lymphocyte infusions or modification of immunosuppressive therapy. In preliminary studies of nonablative allogeneic transplants, many patients with low-grade lymphoma, mantle cell lymphoma, or chronic lymphocytic leukemia have achieved durable remissions.[6,21,22]
These highly sensitive malignancies share several common characteristics. In CML and lymphoma, the malignant cells are derived from antigen-presenting cells-B-lymphocytes in the case of lymphoid malignancies, and dendritic cells generated from CML.[23] Their responsiveness to GVL may, in part, relate to effective in vivo antigen presentation.
• Intermediate Sensitivity to GVL-A second category of malignancies, including AML, multiple myeloma, Hodgkin's disease, and intermediate- grade lymphoma, have intermediate sensitivity to GVL effects. In these diagnoses, allogeneic transplants produce a greater frequency of durable remissions than syngeneic or autologous transplants, but these disorders less frequently respond to donor lymphocyte infusions, and responses are usually transient. Nonablative transplants are most effective for these diagnoses when the malignancy is in a minimal disease state. In patients with resistant or bulky disease, the nonablative or reduced- intensity preparative regimens do not substantially cytoreduce the malignancy, resulting in a high rate of disease recurrence.
• Relatively Insensitive Malignancies-Acute lymphocytic leukemia and high-grade lymphoma appear to be relatively insensitive to GVL effects,[ 24] although patients with GVHD do have a reduced risk of relapse.[3] The malignant lymphoblasts typically lack costimulatory molecules and do not effectively stimulate an immune response.[25] The rapid rate of proliferation of these malignancies may also outpace a developing immune response; only rare patients have responded to donor lymphocyte infusions.
• Graft-vs-Tumor Effects-Graft-vs-tumor effects may also occur against solid tumors, although few studies of allogeneic transplantation in this setting have been performed. Pilot studies in breast cancer have reported antitumor responses in patients with GVHD, suggesting a graftvs- adenocarcinoma effect.[26] Major antitumor responses have been reported in renal cell carcinoma, usually concomitant with the development of acute GVHD.[27] Further studies are required to determine whether graft-vs-tumor effects are sufficiently active to justify the added morbidity related to allogeneic transplantation.
Nonablative and Reduced-Intensity Regimens
In many studies of transplants using nonablative and reduced-intensity regimens, the drugs were chosen for having some activity against the target malignancy as well as providing immunosuppression to prevent graft rejection. Ideally, the regimen would produce some cytoreduction of the malignancy to prevent disease progression long enough to allow an effective graft-vs-malignancy effect to develop. One commonly used regimen- low-dose total body irradiation and fludarabine-produces little myelosuppression and cytoreduction.[ 8] Patients selected for this approach should have either an indolent malignancy or low-bulk disease.
Myeloid Leukemias
Giralt et al initially evaluated the use of standard-dose purine analog- based chemotherapy (fludarabine, 30 mg/m2/d * 4 days; cytarabine, 1 g/m2/d * 4 days; and idarubicin, 12 mg/m2/d * 3 days) as a nonablative preparative regimen in patients with advanced myeloid leukemias.[9] This regimen is truly nonablative, as it has been used to treat AML without transplantation. Nevertheless, it has proved sufficiently immunosuppressive to allow engraftment of matchedsibling transplants. The projected disease-free survival of patients in remission or with < 10% bone marrow blasts at the time of transplant was 40% beyond 2 years. The outcome of patients with refractory leukemia at transplant was poor; < 10% remained in remission at 1 year.
Giralt and colleagues subsequently reported a study combining melphalan (180 mg/m2) and fludarabine (125 mg/m2) for the treatment of advanced acute leukemia.[5] This is a more intensive regimen that should be considered a reduced-toxicity ablative regimen. It is sufficiently immunosuppressive to allow for engraftment from unrelated donors as well as matched siblings. The additional cytoreduction resulted in improved disease-free survival in AML patients, particularly in those receiving transplants in relapse, suggesting that cytoreduction of the malignancy by the preparative regimen is important in AML. Patients with refractory relapse had a 25% extended diseasefree survival, and 56% of patients with chemotherapy-sensitive disease remained in continuous remission beyond 1 year. These results are similar to those achieved with ablative preparative regimens in younger but otherwise similar patients.
Champlin et al reported a comparison of the nonablative fludarabine/ idarubicin/cytarabine regimen with the reduced-intensity ablative regimen of melphalan/fludarabine in older or medically infirm CML patients in late chronic or accelerated phase.[28] The actuarial risk of relapse was only 8% with melphalan/ fludarabine vs 82% with nonablative fludarabine/idarubicin/cytarabine. The melphalan/fludarabine regimen was also more toxic, and relapse-free survival was 37%, compared with 15% for the two groups. These data indicate that cytoreduction of the malignancy by the preparative regimen reduces the risk of relapse in myeloid leukemias.
Slavin and coworkers reported on the use of a reduced-intensity preparative regimen consisting of busulfan (8 mg/kg), fludarabine, and antithymocyte globulin (Thymoglobulin).[ 7,29] Results of this regimen have been particularly encouraging in CML patients. Although less toxic than commonly used full-dose ablative preparative regimens, both the melphalan/fludarabine regimen and busulfan/fludarabine combination produces marked myelosuppression and, therefore, has not been administered without hematopoietic transplantation.
Lymphoma and Hodgkin's Disease
Low-grade lymphomas, mantle cell lymphoma, and chronic lymphocytic leukemia are highly sensitive to graft-vs-malignancy effects. Khouri et al reported that the use of fludarabine (90-125 mg/m2) plus cyclophosphamide (2 g/m2) with or without rituximab (Rituxan) effectively achieves engraftment and durable complete remissions.[6,21,22] A recent study updating this regimen documented extended disease-free survival in 85% of patients with follicular lymphomas and approximately two-thirds of patients with mantle cell lymphoma and transformed large cell lymphomas.[30] These data compare favorably to high-dose cyclophosphamide/ total body irradiation regimens, in which treatment-related mortality rates typically exceed 40%.[31]
Multiple Myeloma
Allogeneic bone marrow transplantation is associated with a high risk of treatment-related mortality in multiple myeloma-in some studies, up to 70%. Use of a nonablative preparative regimen may reduce this morbidity while still inducing a graftvs- myeloma effect.
Giralt et al explored a regimen of melphalan (140 mg/m2) and fludarabine (120 mg/m2) in this setting. In their investigation, 7 of 13 patients with far-advanced myeloma achieved a complete remission.[32] Others have reported similar results.[33-35] Encouraging results have also been reported in patients with Hodgkin's disease.[36,37]
A novel strategy under study in these two diagnoses is the use of tandem autologous and allogeneic transplantation. High-dose therapy with autologous transplant is initially performed to cytoreduce the malignancy, followed by a nonablative allogeneic transplant with the goal of eradicating minimal residual disease. The relative efficacy of this tandem procedure vs a single transplant needs to be determined.
Mechanism of Graft-vs-Malignancy Effects
Clinically, malignancy-specific reactivity can only rarely be demonstrated after allogeneic hematopoietic transplantation. Donor-derived T-cell clones from allogeneic chimeras typically react against both host normal hematopoietic cells and the leukemia cells, suggesting that hematopoietic minor histocompatibility antigens may be targeted. Tumor-specific antigens, such as oligopeptides derived from the junction region of bcr-abl could also be immunogenic.[38]
Over- or abnormally expressed cellular constituents could also serve as a target antigen for GVL. Proteinase 3, a serine protease present in myeloid primary granules, is overexpressed in CML and some cases of AML; it may serve as a target for an antileukemic immune response. Peptide antigens derived from proteinase 3 can stimulate generation of autologous or allogeneic T-cell cytotoxicity against the leukemia.[39] Other candidate overexpressed genes include myeloperoxidase and WT1.
Thus, graft-vs-malignancy could involve broad reactivity overlapping with GVHD, or restricted antihematopoietic or disease-specific responses. The potential target antigens for graftvs- tumor effects are unknown, but overexpressed tissue antigens or polymorphic tissue-restricted minor histocompatibility antigens could be involved. It is also possible that GVHD could produce antitumor effects through cytokines or other mechanisms unrelated to specific Tcell cytotoxicity.
Graft-vs-malignancy responses often occur coincident with or following GVHD. However, some patients achieve a GVL response (ie, remission of their leukemia) without developing GVHD. Although this is consistent with the premise that different target antigens may be involved in each process, responses could result from a greater sensitivity of malignant cells (compared with visceral tissues) to a common immunologic mechanism.
Separating Graft-vs-Malignancy From GVHD
A fundamental goal of allogeneic hematopoietic transplantation is to separate the beneficial GVL effect from the adverse manifestations of GVHD. Given the interplay between GVL and GVHD, the use of posttransplant immunosuppressive therapy can have both positive and negative effects. Immunosuppressive therapy given early posttransplant may inhibit GVL; two randomized studies reported lower relapse rates in AML patients receiving low-dose rather than full-dose cyclosporine (Neoral, Sandimmune) after ablative bone marrow transplantation.[40] A variety of posttransplant regimens have been utilized with nonablative transplants, ranging from full-dose tacrolimus (Prograf) and methotrexate to abbreviated courses of these agents or the combination of cyclosporine and mycophenolate mofetil (CellCept).[35]
As noted, acute GVHD tends to be less severe after a nonablative preparative regimen, but GVHD frequently occurs after early termination of immunosuppressive therapy and is often problematic, particularly in older or debilitated patients. The inclusion of alemtuzumab (Campath) in the regimen has recently been reported to produce a low rate of GVHD.[41] However, it is unclear whether this agent will adversely affect immune recovery or GVL effects after nonmyeloablative transplants.
• Novel Approaches-GVHD is initiated by alloreactive T cells. A novel strategy is to first achieve a tolerated graft and then administer an infusion of engineered T or NK cell populations targeting the malignancy. Possibilities include the use of an initial T-cell-depleted graft or the combination of CD34-positive cells from the donor with nonalloreactive T cells directed against a third party or specific target antigens.[42] Another strategy is to transduce donor T cells with a suicide gene, such as herpes simplex virus-thymidine kinase, which confers sensitivity to ganciclovir (Cytovene) treatment.[43]
An ideal cellular therapy would consist of leukemia-specific effectors devoid of graft-vs-host activity. T-cell clones or lines have been successfully used for the treatment of Epstein-Barr virus-related lymphoproliferative disease and cytomegalovirus infections occurring after allogeneic bone marrow transplantation.[ 44] Falkenburg and coworkers reported a single case of a patient with CML who responded to an infusion of T-cell lines raised against the leukemia.[45] However, the technology involved in producing therapeutic T-cell clones is demanding, and this approach is not sufficiently developed to allow for large-scale clinical trials. Alternatively, T cells reactive against overexpressed malignancy- related antigens could be selected, expanded, and parenterally administered as adoptive cellular therapy.
FIGURE 3
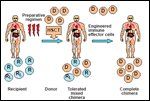
Nonalloreactive Hematopoietic Transplantation
Nonablative hematopoietic transplants may become a platform for the administration of cellular immunotherapy. The goal is to eradicate the malignancy and provide full immune reconstitution without GVHD. One model for optimizing hematopoietic transplantation is summarized in Figure 3. Allogeneic CD34-positive stem cells could be transplanted to achieve engraftment without GVHD. Subsequently, patients could receive engineered T cells or NK cells specific for the malignancy or major infections, devoid of graft-vs-host potential.
Conclusions
The use of relatively nontoxic, nonmyeloablative, or reduced-intensity preparative regimens allows engraftment and the generation of graftvs- malignancy effects. This approach is potentially curative for susceptible malignancies and reduces the risk of treatment-related morbidity. Moreover, this strategy allows the use of allotransplantation in older patients and those with comorbidities that preclude high-dose chemoradiotherapy.
The indications for nonmyeloablative allogeneic transplants need to be defined for each target malignancy. Controlled clinical trials are needed to compare nonablative and reduced-intensity regimens with alternative transplant and nontransplant strategies.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Thomas ED: Bone marrow transplantation for malignant disease. J Clin Oncol 1:517, 1983.
2. Gale RP, Champlin RE: How does bone marrow transplantation cure leukemia? Lancet 2:28-30, 1984.
3. Sullivan KM, Weiden PL, Storb R, et al: Influence of acute and chronic graft-versushost disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood 73:1720-1728, 1989.
4. Champlin R, Khouri I, Shimoni A, et al: Harnessing graft-versus-malignancy: Non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol 111:18-29, 2000.
5. Giralt S, Thall PF, Khouri I, et al: Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 97:631-637, 2001.
6. Khouri I, Keating M, Korbling M, et al: Transplant Lite: induction of graft-versus-leukemia using fludarabine-based nonablative chemotherpy and allogeneic blood progenitor cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 16:2817-2824, 1998.
7. Slavin S, Nagler A, Naparstek E, et al: Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 91:756-763, 1998.
8. McSweeney PA, Niederwieser D, Shizuru JA, et al: Hematopoietic cell transplantation in older patients with hematologic malignancies:Replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 97:3390- 3400, 2001.
9. Giralt S, Estey E, Albitar M, et al: Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: Harnessing graft-versus-leukemia without myeloablative therapy. Blood 89:4531-4536, 1997.
10. Childs R, Clave E, Contentin N, et al: Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: Full donor T-cell chimerism precedes alloimmune responses. Blood 94:3234-3241, 1999.
11. Couriel D, Giralt S, De Lima M, et al: Graft-versus-host disease after non-myeloablative versus myeloablative conditioning regimens in fully matched sibling donor hematopoietic stem cell transplants. Blood 96:408a, 2000.
12. Ferrara JLM, Deeg HJ: Mechanisms of disease: Graft-versus-host disease. N.Engl.J.Med. 324: 667-674, 1991
13. Martino R, Caballero MD, Canals C, et al: Reduced-intensity conditioning reduces the risk of severe infections after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplantation 28:341-347, 2001.
14. Clift RA, Buckner CD, Appelbaum FR, et al: Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: A randomized trial of two irradiation regimens. Blood 77:1660-1665, 1991.
15. Clift RA, Buckner CD, Appelbaum FR, et al: Long-term follow-up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood 92:1455-1456, 1998.
16. Slattery JT, Clift RA, Buckner CD, et al: Marrow transplantation for chronic myelogenous leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 89:3055-3060, 1997.
17. Gale RP, Horowitz MM, Ash RC, et al: Identical-twin bone marrow transplants for leukemia. Ann Intern Med 120:646-652, 1994.
18. Horowitz MM, Gale RP, Sondel PM, et al: Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75:555-562, 1990.
19. Kolb HJ, Schattenberg A, Goldman JM, et al: Graft-vs-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 86:2041-2050, 1995.
20. Van Besien KW, De Lima M, Giralt SA, et al: Management of lymphoma recurrence after allogeneic transplantation: The relevance of graft-versus-lymphoma effect. Bone Marrow Transplantation 19:977-982, 1997.
21. Khouri I, Lee M-S, Romaguera J, et al: Allogeneic hematopoietic transplantation for mantle-cell lymphoma: Molecular remissions and evicence of graft-versus-malignancy. Ann Oncol 10:1293-1299, 1999.
22. Khouri I, Saliba R, Giralt S, et al: Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: Low incidence of toxicity, graftvs- host disease and treatment-related mortality. Blood 98:3595-3599, 2001.
23. Choudhury A, Gajewski J, Liang JC, et al: Use of leukemic dendritic cells for the generation of anti-leukemic cellular cytotoxicity against Philadelphia chromosome positive chronic myelogenous leukemia. Blood 89:1133-1142, 1997.
24. Collins RH Jr, Goldstein S, Giralt S, et al: Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplantation 26:511-516, 2000.
25. Stripecke R, Cardoso AA, Pepper KA, et al: Lentiviral vectors for efficient delivery of CD80 and granulocyte-macrophage-colonystimulating factor in human acute lymphoblastic leukemia and acute myeloid leukemia cells to induce antileukemic immune responses. Blood 96:1317-1326, 2000.
26. Ueno NT, Rondón G, Mirza NQ, et al: Allogeneic peripheral-blood progenitor-cell transplantation for poor-risk patients with metastatic breast cancer. J Clin Oncol 16:986-993, 1998.
27. Childs R, Chernoff A, Contentin N, et al: Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheralblood stem-cell transplantation. N Engl J Med 343:750-758, 2000.
28. Champlin RE, Cohen A, Saliba R, et al: Dose matters; improved disease control with increased cytoreduction in nonablative bmt for late chronic or accelerated phase CML. Blood. In press.
29. Nagler A, Aker M, OR R, et al: Lowintensity conditioning is sufficient to ensure engraftment in matched unrelated bone marrow transplantation. Exper Hematol 29:362- 370, 2001.
30. Khouri I, Champlin RE: Non-myeloablative stem cell transplantation for lymphoma. Semin Hematol. In press.
31. Van Besien K, Sobocinski K, Rowlings PA, et al: Allogeneic bone marrow transplantation for low-grade lymphoma. Blood 92:1832- 1836, 1998.
32. Giralt SA, Weber D, Aleman A, et al: Nonmyeloablative conditioning with fludarabine/ melphalan for patients with multiple myeloma (abstract). Blood 94(suppl 1):1549a, 1999.
33. Badros A, Barlogie B, Morris C, et al: High response rate in refractory and poor-risk multiple myeloma after allotransplantation using a nonmyeloablative conditioning regimen and donor lymphocyte infusions. Blood 97:2574-2579, 2001.
34. Garban F, Attal M, Rossi JF, et al: Immunotherapy by non-myeloablative allogeneic stem cell transplantation in multiple myeloma: results of a pilot study as salvage therapy after autologous transplantation. Leukemia 15:642- 646, 2001.
35. McSweeney P, Niederwieser D, Shizuru J, et al: Outpatient allografting with minimally myeosuppressive immunosuppressive conditioning of low-dose TBI and postgrafting cyclosporine and mycophenolate mofetil (abstract). Blood 94(suppl 1):393a, 1999.
,b>36. Anderlini P, Giralt S, Andersson B, et al: Allogeneic stem cell transplantation with fludarabine- based, less intensive conditioning regimens as adoptive immunotherapy in advanced Hodgkin’s disease. Bone Marrow Transplantation 26:615-620, 2000.
37. Carella AM, Cavaliere M, Lerma E, et al: Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin’s disease and non-Hodgkin’s lymphoma. J Clin Oncol 18:3918-3924, 2000.
Pinilla-Ibarz J, Cathcart K, Korontsvit T, et al: Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates specific immune responses. Blood 95:1781-1787, 2000.
39. Molldrem JJ, Lee PP, Wang CQ, et al: Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nature Med 6:1018-1023, 2000.
40. Bacigalupo A, Lamparelli T, Gualandi F, et al: Increased risk of leukemia relapse with high dose cyclosporine after allogeneic marrow transplantation for acute leukemia: 10 year follow-up of a randomized study. Blood 98:3174-3175, 2001.
41. Chakraverty R, Peggs K, Chopra R, et al: Limiting transplantation-related mortality following unrelated donor stem cell transplantation by using a nonmyeloablative conditioning regimen. Blood 99:1071-1078, 2002.
42. Reich-Zeliger S, Zhao Y, Krauthgamer R, et al: Anti-third party CD8+ CTLs as potent veto cells: Coexpression of CD8 and fasL is a prerequisite. Immunity 13:507, 2000.
43. Bonini C, Ferrari G, Verzeletti S, et al: HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 276:1719-1724, 1997.
44. Heslop H, Ng C, Li C, et al: Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of genemodified virus-specific T lymphocytes. Nature Med 2:551, 1996.
45. Falkenburg JHF, Wafelman AR, Joosten P, et al: Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood 94:1201-1208, 1999.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.