Novel Therapeutics Build on Irinotecan-based Treatment
PEAPACK, New Jersey-Potential directions for irinotecan (Camptosar) clinical research in the future and for the application of novel therapeutics were presented by Langdon Miller, MD, of Pharmacia Oncology Development in Peapack, New Jersey.
PEAPACK, New JerseyPotential directions for irinotecan (Camptosar) clinical research in the future and for the application of novel therapeutics were presented by Langdon Miller, MD, of Pharmacia Oncology Development in Peapack, New Jersey.
"The developmental objective in colorectal cancer is to improve the therapeutic index in metastatic disease. This will include developing new schedules for treatment of older patients, diarrhea control, and pharmacogenomically directed therapy," Dr. Miller said.
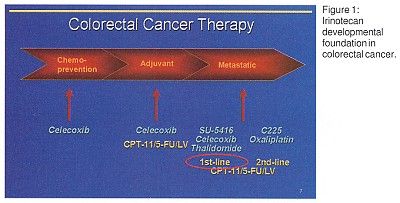
In addition, new agents are being tested for their activity against colorectal and other forms of cancer. These agents include celecoxib (Celebrex), SU-5416, and C225. "From our foundation in colorectal cancer (Figure 1), we see these novel therapeutics standing on the shoulders of irinotecan-based therapy for metastatic and adjuvant treatment," Dr. Miller said. "Celecoxib in particular has the prospect of extending our reach into the exciting area of cancer prevention."
Use in Older Patients
Dr. Miller said that first-line use of irinotecan/fluorouracil (5-FU)/leucovorin is highly age-dependent, with only 35% of eligible patients over age 65 receiving treatment. Pharmacia researchers retrospectively analyzed data with respect to age and found no age-related differences in time to progression or in survival.
Diarrhea was slightly but not dramatically more common in the older patients, as was neutropenia. "Older patients may be a little more sensitive to toxicity, but dosage modification takes care of these problems," Dr. Miller said.
Researchers will also be evaluating a new schedule that includes irinotecan/5-FU/leucovorin on weeks 1 and 2 every 3 weeks.
Additional Studies
Dr. Miller said that diarrhea prevention agents of primary interest are celecoxib and thalidomide (Thalomid). A diarrhea prevention study of thalidomide is being developed.
Other studies will evaluate the survival benefits of the experimental agent SU-5416 in patients with first-line metastatic colorectal cancer. In one trial, patients will receive irinotecan/5-FU/leucovorin and then be randomized to receive or not receive SU-5416. In the other trial, patients will receive 5-FU/leucovorin with or without SU-5416.
Dr. Miller also reported that a celecoxib adjuvant study is planned for patients with high-risk stage III colorectal cancer.
Studies in other cancers include irinotecan in first-line therapy of pancreatic, gastric, and small-cell lung cancer.
Two phase III trials-one ongoing and one planned-test different schedules of cisplatin (Platinol)/irinotecan in patients with small-cell lung cancer. Pharmacia Oncology Development is conducting the ongoing trial and the Southwest Oncology Group (SWOG) is planning the other trial.
A gemcitabine (Gemzar)/irinotecan combination therapy has been studied in a phase II trial in pancreatic cancer, and researchers have correlated response with levels of CA 19-9. "There appears to be an amazing correlation between CA 19-9 response and tumor response," Dr. Miller said.
Based on the phase II results, a phase III study of gemcitabine/irinotecan is now ongoing. "We hope that CA 19-9 can become an accepted study endpoint if it works out in this phase III trial," Dr. Miller stated.