Panitumumab Effective in Heavily Pretreated Metastatic CRC
ORLANDO-Panitumumab,which binds to the epidermalgrowth factor receptor (EGFR), “demonstratedencouraging antitumor ac
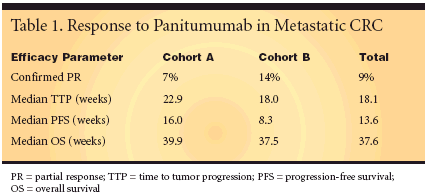
ORLANDO-Panitumumab,which binds to the epidermalgrowth factor receptor (EGFR), "demonstratedencouraging antitumor activityin patients with metastatic carcinomaof the colon or rectum whofailed standard chemotherapy," ImitiazMalik, MD, said (abstract 3520).Dr. Malik reported data from anongoing phase II study of 148 patientswho had treatment-resistant metastaticcolorectal cancer (CRC) despite priortreatment with a fluoropyrimidine(± leucovorin) and either irinotecan(Camptosar) or oxaliplatin (Eloxatin),or both. Endpoints included tumorresponse (according to RECIST criteria),time to disease progression, survival,and safety.Patients were stratified accordingEGFR status. Cohort A included pa-tients with EGFR staining of 2+ or 3+in at least 10% of tumor cells. CohortB included patients with EGFR stainingof the sum of 1+, 2+, and 3+ in10% of tumor cells but with the sumof 2+ and 3+ in < 10% of tumor cells.Panitumumab was given intravenouslyat 2.5 mg/kg once weekly in 8-week cycles. Treatment continued untildisease progression or unacceptabletoxicity occurred.Mature Data ConfirmPrevious FindingPanitumumab was active in bothpatient cohorts (Table 1). The fourmost frequent grade 3 or 4 side effectswere rash, fatigue, vomiting, nausea,and pruritus. One hundred forty-onepatients (95%) had skin toxicity, including5% that were grade 3. Dr.Malik said that one infusion-relatedgrade 3 adverse event, which did notrequire dose modification, was reported,and there were no human antihumanantibodies in the 145 patientstested."These mature data confirm previouslyreported safety and responsefindings and provide encouraging survivaldata in patients with metastaticCRC who have failed multiple lines ofstandard chemotherapy," Dr. Maliksaid. "Median duration of responsewas 18.1 weeks by central review, andresponse was seen in patients receivingup to four lines of prior therapy.Also, there appeared to be no significantdifferences in all efficacy parametersassessed between cohort A andcohort B."
Dr. Malik said that panitumumabis currently being studied in a phase IItrial as third- or fourth-line treatmentin patients with metastatic CRC whosetumors have low or negative EGFRexpression. The antibody is also inrandomized trials in combination withbevacizumab (Avastin) and chemotherapyas first-line therapy.This study was supported by Amgen,Inc.