Percutaneous Ablation of Kidney Tumors in Nonsurgical Candidates
Although resection currently remains the standard of care for renalcarcinoma, the search for less invasive treatments has led to alternativesurgical approaches. Even less invasive, and appropriate for manygroups of patients, is percutaneous radiofrequency ablation, which inducestumor necrosis via lethal hyperthermia. Multiple series of renaltumors treated with percutaneous ablation in vivo and left in situ havebeen published; these series reveal that for small renal tumors,radiofrequency ablation results in complete necrosis at imaging in 79%to 100% of cases. Because current results come from tumors left in situwith short postablation follow-up, long-term results are necessary tocompare outcomes to surgical standards. Complication rates are lowerthan those following partial nephrectomy. Future reports will shed lighton the long-term outcomes of percutaneous ablation and the relativeadvantages and disadvantages of various technologies for thermal ablation.
Although resection currently remains the standard of care for renal carcinoma, the search for less invasive treatments has led to alternative surgical approaches. Even less invasive, and appropriate for many groups of patients, is percutaneous radiofrequency ablation, which induces tumor necrosis via lethal hyperthermia. Multiple series of renal tumors treated with percutaneous ablation in vivo and left in situ have been published; these series reveal that for small renal tumors, radiofrequency ablation results in complete necrosis at imaging in 79% to 100% of cases. Because current results come from tumors left in situ with short postablation follow-up, long-term results are necessary to compare outcomes to surgical standards. Complication rates are lower than those following partial nephrectomy. Future reports will shed light on the long-term outcomes of percutaneous ablation and the relative advantages and disadvantages of various technologies for thermal ablation.
Over 35,000 new cases of renal cell carcinoma (RCC) occurred in the United States in 2004,[1] most of them detected as incidental imaging findings on computed tomography (CT), magnetic resonance (MR), or ultrasound.[2,3] Since most of these tumors are relatively small when detected, the classic clinical triad of flank pain, hematuria, and palpable mass is now rarely encountered. Many of these incidentally discovered RCCs are also slowly growing. Bosniak et al showed that RCCs smaller than or equal to 3.5 cm grow at an average rate of 0 to 1.1 cm/yr (mean 0.36 cm/yr).[4] Most RCCs are surgically removed, and resection of RCC remains the standard of care.[5] Traditional treatment has been open complete nephrectomy, but the search for less invasive procedures as well as for nephronsparing surgery has led to alternative surgical approaches.[6,7] These include partial open and laparoscopic partial or complete nephrectomy. For appropriately selected tumors, nephron- sparing resection has shown outcomes that are equivalent to total nephrectomy.[5-7] Open, partial, and laparoscopic nephrectomies are now available to most patients. For some patients, however, an even less invasive procedure may be indicated. Patients with solitary kidneys or limited renal function, for example, may not tolerate even a partial nephrectomy without requiring postoperative dialysis. For patients with bilateral or multiple RCCs, further partial nephrectomy may tip them to dialysis. Finally, patients with extensive comorbid conditions may face unacceptable risks from surgery and general anesthesia. Imaging surveillance has been advocated for elderly patients with comorbid conditions placing them at high risk for operative complications or complications related to anesthesia, because of the slow growth of these small tumors and the strong likelihood that many of them will never become clinically significant. This course of action may result in unacceptable patient anxiety, however. Percutaneous ablation may provide an alternative treatment option for all these groups of patients. Ablation of RCC left in situ has been performed intraoperatively using cryoablation before percutaneous cryoprobes became available.[8] Cryoablation induces tumor necrosis by freezing tissue. The first needle applicators suitable for percutaneous application were radiofrequency electrodes. Thus, most of the published literature on percutaneous ablation of renal masses is on RF ablation, which induces tumor necrosis via lethal hyperthermia. Now that percutaneous cryoprobes are available, experience with imageguided percutaneous cryoablation will undoubtedly increase. A microwave ablation system with percutaneous applicators, also based on hyperthermia, has become available in the United States, but no published reports of its use in renal tumors left in situ have yet appeared. In 1997, Zlotta et al reported the first percutaneous application of renal RFA, confirming its safety and feasibility under local analgesia.[9] In his initial series, three tumors were subsequently resected, confirming RFA's ability to create extensive local necrosis in in vivo renal tumors.[9] In 1999, McGovern et al reported the first in situ renal tumor treated solely with RFA.[10] In 2000, Gervais et al presented the first series of such patients treated solely with RFA.[11] Nine tumors in eight patients underwent RFA with postablation imaging surveillance to monitor treatment response. Because the tumors were left in situ, assessment of tumor response was performed with imaging. Gervais et al applied a CT or MR interpretive scheme based on extrapolation of radiologic- pathologic correlation work in liver tumor ablations performed by Goldberg et al.[11,12] In this study, necrosis was shown to correlate with lack of enhancement to within 2 mm and tumor enhancement was shown to correlate with viable tumor.[12] This interpretative scheme has since been widely applied by other investigators. Since these early reports, multiple series of renal tumors treated with percutaneous ablation in vivo and left in situ have been published.[13-26] Table 1 summarizes the results of the larger published series of percutaneous RFA of renal tumors. These series reveal that for small renal tumors, RFA results in complete necrosis at imaging in 79% to 100% of cases.[13- 26] Differences in results are related partly to differences in protocol. In one protocol in which early residual disease was treated with repeat percutaneous ablation, for example, 100% complete necrosis at imaging was achieved for all tumors 4 cm or smaller.[ 13] The series with the lowest success rate (79%) used less powerful generators than are now commonly available, perhaps resulting in less complete ablation.[19] Modern systems average over 90% complete necrosis in small tumors. The follow-up period following renal tumor ablation has been short, however, averaging less than 3 years. Although early experience is encouraging, long-term outcomes are necessary to provide meaningful comparison to standard surgical resection.
Fewer published reports on percutaneous cryoablation exist, but laparoscopic experience suggests we can expect similar outcomes.[8,27] Gill et al reported on 56 patients, 36 with RCC, who underwent laparoscopic renal cryoablation with residual/recurrent RCC at biopsy in two tumors.[8] Enhancement characteristics were not reported, but 17 tumors had disappeared by 3 years.[8] In a smaller percutaneous series, Shingleton and Sewell performed MR-guided cryoablation in 14 patients with 15 renal masses in solitary kidneys, two of whom were lost to follow-up.[27] Of the remaining 12 patients, 10 (83%) underwent complete necrosis at imaging, with a mean of 17 months of follow-up.[27] Case Evaluation
Case selection for percutaneous ablation involves consideration of three broad issues: patient factors, tumor factors, and feasibility factors. Patient Factors
Factors that define the clinical indications for percutaneous ablation of RCC include comorbid conditions rendering surgery risky, limited renal function, and multiple RCC or its predisposition as with von Hippel-Lindau disease or familial types of RCC.[13] Patient evaluation is best done in consultation with a urologist experienced in treatment of RCC to ensure that all surgical options have been considered. A collaborative approach consisting of a multidisciplinary team with members from urology and interventional radiology provides the best combined expertise for successful patient management and outcomes.[13] Tumor Factors
Tumor size and location must be considered. Small tumors are ideal because of the technical limitations of the ablation systems with respect to the volume of tissue that undergoes necrosis. The size criterion for inclusion of a "small" tumor in RFA has ranged from 2.5 to 4 cm depending on the series.[13-26] A recent RCC analysis based on 100 cases suggests that 4 cm is an appropriate definition, with all tumors 4 cm or smaller undergoing complete necrosis, 92% (48/ 52) in one ablation session and the remaining 8% in two sessions.[13] Larger tumors have been completely ablated in some series, but experience with these is less extensive,[13] and increasing size often requires an increasing number of ablation sessions.[ 13] The largest tumor completely treated with percutaneous ablation alone was 5.5 cm.[19] Some have combined embolization with ablation for larger tumors.[21,22] Tumor location also affects ablation results. The ideal renal tumor for percutaneous ablation is an exophytic RCC. The surrounding perirenal fat serves as an insulator to allow higher temperatures of longer duration to be achieved with RFA. Central tumors, on the other hand, can prove more difficult to ablate because of proximity to large hilar vessels. Blood flow provides constant cooling during RFA, thus limiting the effect of the ablation. Gervais et al demonstrated that central location did not necessarily preclude complete ablation, but central tumors as a group were less likely to undergo complete necrosis compared with exophytic tumors.[13] Feasibility
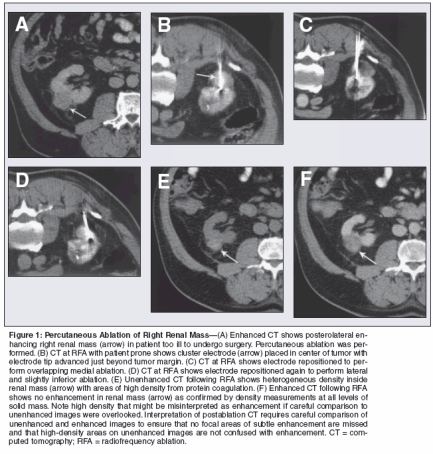
Case evaluation assesses whether the case is feasible and safe using a percutaneous approach. A safe percutaneous path to the tumor, avoiding bowel and large vessels, must exist. Even if a percutaneous approach is possible with the needle applicator, the proximity of the tumor to other structures that might be damaged by thermal injury must be considered. For renal tumors, this means careful attention to the location of bowel, ureter, and ureteropelvic junction. Even when these structures are adjacent to tumor or within a few millimeters, percutaneous ablation may still be possible if the structures can be separated from tumor via positional maneuvers, hydrodissection, instillation of CO2, balloon displacement, or manual compression.[14,28,29] For tumors that cannot be separated from vital structures, surgical approaches to ablation or resection or imaging follow-up remain options. Performing Ablation Planning and performing a renal tumor ablation requires diligent attention to the findings on preablation diagnostic imaging. Tumor margins must be known to plan the desired size of the ablation zone. Pavlovich et al emphasized preservation of normal renal parenchyma in their early series.[ 19] This technique may in part have been responsible for their lower rate of complete ablation. Although achievement of a 5- to 10-mm margin of normal renal tissue at the RCC/kidney interface is not mandatory, Ogan et al have advocated striving for a small margin of renal tissue when performing ablation to help ensure more complete tumor necrosis.[ 23] An approach that includes margins in the planned ablation zone is further supported by the work of Goldberg et al, who found that viable tumor cells remained present at the margins in four of five liver tumors where the zone of ablation was equal in size to the tumor in the absence of enhancement at CT.[12] These spatial resolution limitations of imaging technology must be considered when planning and performing percutaneous ablation. Although some have advocated performing the first RFA at the tumor/kidney interface to devascularize this area, recent work suggests that this approach does not produce outcomes different from other approaches.[14] A single applicator may not result in an ablation zone of adequate size, or the geometry of the ablation zone may not correspond to the tumor geometry, leaving viable tumor. For this reason, multiple applications are necessary. Cryoablation uses multiple single cryoprobes, placed and activated simultaneously, until multiple freezethaw cycles are completed. On the other hand, most RF electrode systems are applied as a single applicator with multiple overlapping ablations performed in one session by repositioning of the electrode after each ablation to cover the entire tumor volume (Figure 1). New technology allows up to three simultaneous electrode placements, with the generator delivering power to each electrode sequentially.[ 30]
Computed tomography, MR, or ultrasound can provide imaging guidance for positioning of the needle applicators.[ 13,25,27] Each has its advantages and disadvantages. Magnetic resonance can provide accurate assessment of the treatment margin in multiple planes while the patient is still on the table, but it is expensive, not widely available for interventional use, and requires special monitoring equipment as well as special MRcompatible applicators. Ultrasound can provide rapid real-time evaluation of needle position without the use of ionizing radiation, but it may not easily demonstrate some tumors, particularly on the left. Echoes generated by RFA and the echogenic proximal edge of the iceball at cryoablation preclude accurate monitoring of the treatment effect and limit visibility for subsequent repositioning of RF applicators for overlapping ablations. Computed tomography provides accurate localization of the needle applicators, and visibility of the applicators is not limited by treatment effects such as gas formation or small amounts of hemorrhage. Neither ultrasound nor noncontrast CT predicts the precise location of the treatment margin. Near the end of an ablation, a bolus of contrast material may be useful in demonstrating remaining tumor. Contrast material may concentrate near the ablation zone, however, limiting the utility of subsequent boluses of contrast material. Radiofrequency systems allow for electrocautery of the needle tract upon removal. Tract ablation theoretically limits tract seeding and the risk of hemorrhage. Patient Management
Issues to consider in preparing a patient for ablation include tissue diagnosis, status of outpatient vs shortstay inpatient care, and choice of sedation vs anesthesia. Percutaneous ablation of RCC can be performed as an outpatient procedure,[13] but admission may be required to allow complete recovery from sedation. Intravenous sedation is used in many cases (midazolam 2 to 5 mg, fentanyl 100 to 300 μg, meperidine 50 to 100 mg).[13] Selected patients may require general anesthesia if they do not meet institutional criteria for IV sedation. Some institutions prefer to perform all cases under general anesthesia.[18] Differential diagnosis of a solid enhancing mass on CT or MRI includes RCC, fat poor angiomyolipoma, oncocytoma, metastases, or lymphoma. Definitive pathologic diagnosis of a renal mass left in situ requires needle biopsy. Traditionally, for management purposes, urologists have treated a solid enhancing renal mass at CT or MR as renal cell carcinoma. Since needle biopsies may miss RCC in a small number of cases, all these masses were resected, providing material for histology. Leaving the renal tumor in situ after ablation, however, provides no tissue diagnosis to help guide postablation management. A recent report found that 10 of 27 patients referred for ablation had benign masses.[31] Thus, most patients undergo needle biopsy to establish a diagnosis prior to ablation. In selected cohorts, such as patients with von Hippel-Lindau, some have argued against biopsy prior to ablation because the likelihood of RCC is high regardless of biopsy results.[19] This area remains controversial. Following ablation, imaging follow- up with unenhanced and enhanced CT or MR allows for response assessment (Figures 1E and 1F).[11] Although imaging intervals vary among institutions, most radiologists perform imaging within one month for prompt detection of residual viable tumor.[13-27] Small foci of residual viable tumor can then be ablated again.[14] Because of the lack of longterm data on the efficacy of ablation, imaging continues indefinitely, initially at 3- to 6-month intervals and then at annual intervals once no viable tumor has been confirmed in the first year or two of follow-up. Recovery from percutaneous ablation involves the effects of sedation or anesthesia on the first day. In the first few days, patients normally experience mild to moderate local discomfort that improves over time. Postablation syndrome, flulike symptoms such as fever, muscle ache, and fatigue, is seen in a minority of patients. The syndrome is self-limiting and responds well to acetaminophen or nonsteroidal anti-inflammatory agents. Complications are rare following RFA compared with partial nephrectomy.[ 5-7,13] The most common is hemorrhage, and this may be more common in central tumors.[13] Urinary collecting system obstruction can occur from ureteral injury with stricture formation or from bleeding into the collecting system.[13,14] Injury to nerves from the lumbar plexus may cause transient paresthesias along cutaneous nerve distributions.[13,15,19] Tract seeding has been reported in a single case in which a cutaneous tumor was subsequently removed.[17] There is also potential for bowel injury or pleural complication requiring thoracostomy drainage. Conclusion Percutaneous ablation of renal tumors has shown promising early results. Until long-term data are available, the use of percutaneous ablation for solid renal masses is limited to patients who are not ideal candidates for surgical resection. Tumor factors play an important role in case selection, with tumors 4 cm or smaller yielding up to 100% complete necrosis with modern ablation systems. Exophytic location provides a favorable "oven effect" from the insulating properties of the perirenal fat, while central location may make complete ablation more difficult due to the perfusion in large hilar blood vessels. Because current results come from tumors left in situ with short postablation follow-up, longterm results are necessary to compare outcomes to surgical standards. Complication rates are lower than those following partial nephrectomy. Future reports will shed light on the long-term outcomes of percutaneous ablation and the relative advantages and disadvantages of various technologies for thermal ablation.
Disclosures:
Dr. Gervais is a consultant for Valleylab. Dr. Arellano and Dr. Mueller have no significant financial arrangement or affiliation with any manufacturer of any pharmaceutical or medical device and are not affiliated in any manner with any provider of any commercial medical or health-care professional service.
References:
1. Jemal A, Tiwari RC, Murray T, et al: Cancer statistics, 2004. CA Cancer J Clin 54:8-29, 2004.
2. Smith SJ, Bosniak MA, Megibow AJ, et al: Renal cell carcinoma: Earlier discovery and increased detection. Radiology 170:699-703, 1989.
3. Jayson M, Sanders H: Increased incidence of serendipitously discovered renal cell carcinoma. Urology 51:203-205, 1998.
4. Bosniak MA, Birnbaum BA, Krinsky GA, et al: Small renal parenchymal neoplasms: Further observations on growth. Radiology 97:589- 597, 1995.
5. Novick AC, Campbell SC: Renal tumors, in Walsh PC (ed): Campbell’s Urology, 8th ed, pp 2672-2731. Philadelphia, Saunders, 2002.
6. Uzzo RG, Novick AC: Nephron sparing surgery for renal tumors: Indications, techniques and outcomes. J Urol 166:6-18, 2001.
7. Reddan DN, Raj GV, Polascik TJ: Management of small renal tumors: An overview. Am J Med 110:558-562, 2001.
8. Gill IS, Remer EM, Hasan WA, et al: Renal cryoablation: Outcome at 3 years. J Urol 173:1903-1907, 2005.
9. Zlotta AR, Wildshutz T, Raviv G, et al: Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: Ex vivo and in vivo experience. J Endourol 11:251-258, 1997. https:/ /phsxchweb.partners.org/exchweb/bin/ redir.asp?URL=http://radiology.rsnajnls.org/ c g i / e x t e r n a l _ r e f ? a c c e s s _ n u m = 9376843%26link_type=MED
10. McGovern FJ, Wood BJ, Goldberg SN, et al: Radiofrequency ablation of renal cell carcinoma via image guided needle electrodes. J Urol 161:599-600, 1999. https:// phsxchweb.partners.org/exchweb/bin/ redir.asp?URL=http://radiology.rsnajnls.org/ c g i / e x t e r n a l _ r e f ? a c c e s s _ n u m = 9915457%26link_type=MED
11. Gervais DA, McGovern FJ, Wood BJ, et al: Radio-frequency ablation of renal cell carcinoma: Early clinical experience. Radiology 217:665-672, 2000.
12. Goldberg SN, Gazelle GS, Compton CC, et al: Treatment of intrahepatic malignancy with radiofrequency ablation: Radiologicpathologic correlation. Cancer 88:2452-2463, 2000.
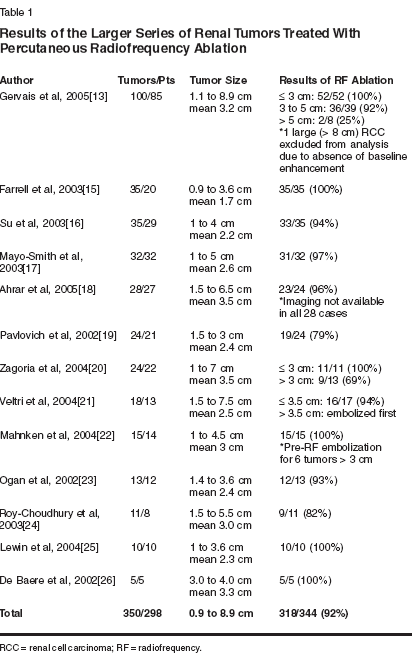
13. Gervais DA, McGovern FJ, Arellano RS, et al: Radiofrequency ablation of renal cell carcinoma part 1: Indications, results, and role in patient management over a six year period and ablation of 100 tumors. Am J Roentgenol 185:64-71, 2005.
14. Gervais DA, Arellano RS, McGovern FJ, et al: Radiofrequency ablation of renal cell carcinoma part 2: Lessons learned with ablation of 100 tumors. Am J Roentgenol 185:72-80, 2005.
15. Farrell MA, Charboneau WJ, DiMarco DS: Imaging-guided radiofrequency ablation of solid renal tumors. Am J Roentgenol 180:1509-1513, 2003.
16. Su LM, Jarrett TW, Chan DY, et al: Percutaneous computed tomography-guided radiofrequency ablation of renal masses in high surgical risk patients: Preliminary results. Urology 61(4 suppl 1):26-33, 2003.
17. Mayo-Smith WW, Dupuy DE, Parikh PM, et al: Imaging-guided percutaneous radiofrequency ablation of solid renal masses: Technique and outcomes of 38 treatment sessions in 32 consecutive patients. Am J Roentgenol 180:1503-1508, 2003.
18. Ahrar K, Matin S, Wood CG, et al: Percutaneous radiofrequency ablation of renal tumors: Technique, complications, and outcomes. J Vasc Interv Radiol 16:679-688, 2005.
19. Pavlovich CP, Walther MM, Choyke PL, et al: Percutaneous radio frequency ablation of small renal tumors: Initial results. J Urol 167:10-15, 2002.
20. Zagoria RJ, Hawkins AD, Clark PE, et al: Percutaneous CT-guided radiofrequency ablation of renal neoplasms: Factors influencing success. Am J Roentgenol 183:201-207, 2004.
21. Veltri A, De Fazio G, Malfitana B, et al: Percutaneous US-guided RF thermal ablation for malignant renal tumors: Preliminary results in 13 patients. Eur Radiol 14:2303-2310, 2004.
22. Mahnken AH, Rohde D, Brkovic D, et al: Percutaneous radiofrequency ablation of renal carcinoma: Preliminary results. Acta Radiol 46:208-214, 2005.
23. Ogan K, Jacomides L, Dolmatch BL, et al: Percutaneous radiofrequency ablation of renal tumors: Technique, limitations, and morbidity. Urology 60:954-958, 2002.
24. Roy-Choudhury SH, Cast JEI, Cooksey G, et al: Early experience with percutaneous radiofrequency ablation of small solid renal masses. Am J Roentgenol 180:1055-1061, 2003.
25. Lewin JS, Nour SG, Connell CF, et al: Phase II clinical trial of interactive MR imaging- guided interstitial radiofrequency thermal ablation of primary kidney tumors: Initial experience. Radiology 232:835-845, 2004.
26. de Baere T, Kuoch V, Smayra T, et al : Radiofrequency ablation of renal cell carcinoma: Preliminary clinical experience. J Urol 167:1961-1964, 2002.
27. Shingleton WB, Sewell PE: Cryoablation of renal tumors in patients with solitary kidneys. BJU Int 92:237-239, 2003.
28. Kam AW, Littrup, Walther MM, et al: Thermal protection during percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol 15:753-758, 2004.
29. Farrell MA, Charboneau JW, Callstrom MR, et al: Paranephric water instillation: A technique to prevent bowel injury during percutaneous renal radiofrequency ablation. Am J Roentgenol 181:1315-1317, 2003.
30. Haemmerich D, Lee FT Jr, Schutt DJ, et al: Large-volume radiofrequency ablation of ex vivo bovine liver with multiple cooled cluster electrodes. Radiology 234:563-568, 2005.
31. Tuncali K, vanSonnenberg E, Shankar S, et al : Evaluation of patients referred for percutaneous ablation of renal tumors: Importance of a preprocedural diagnosis. Am J Roentgenol 183:575-582, 2004.