Pharmacogenetics Profiling Expected to Transform Drug Therapy
CHICAGO-Obtaining an individual patient’s genetic profile for genetic polymorphisms known to affect drug responsiveness or risk of drug toxicity will become a routine part of medical care over the next 25 years and will dramatically transform the prescribing process, Mark J. Ratain, MD, predicted at the Vanderbilt University Symposium. Dr. Ratain is professor of medicine and chairman of the Committee on Clinical Pharmacology at the University of Chicago.
CHICAGOObtaining an individual patient’s genetic profile for genetic polymorphisms known to affect drug responsiveness or risk of drug toxicity will become a routine part of medical care over the next 25 years and will dramatically transform the prescribing process, Mark J. Ratain, MD, predicted at the Vanderbilt University Symposium. Dr. Ratain is professor of medicine and chairman of the Committee on Clinical Pharmacology at the University of Chicago.
"Determining the patient’s genomic sequence will soon become a part of standard practice and will affect both selection of drugs and selection of dosages," Dr. Ratain said. "The cost is now under $1 for each genotype, and I expect that cost to drop quickly as medical databases that relate genotypes to effects on drug function become available. I think therapeutics will be very different 25 years from now," Dr. Ratain said.
Several clues point to genetic polymorphisms in the metabolism of a particular drug, Dr. Ratain noted. These include a high coefficient of variance (CV), unpredictable toxicity, evidence for bimodality in either pharmacokinetics or toxicity, and possible or known metabolism by enzymes associated with other polymorphisms. The fact that adverse reactions have been seen in over half of patients taking irinotecan (Camptosar), including grade 3/4 late-onset diarrhea in about 30%, suggested that a genetic polymorphism might be a contributory factor.
Points of Influence
Irinotecan metabolism offers several possible points at which a genetic polymorphism might exert influence. Dr. Ratain said that plasma disposition curves following irinotecan infusion show significant individual variability, particularly with regard to levels of the glucuronide metabolite SN-38G. In general, patients who maintain higher levels of SN-38G are less likely to have problems with diarrhea. Those whose metabolisms are slower to convert SN-38 to the glucuronide form have more diarrhea.
This observation led to construction of a model to explain irinotecan pharmacokinetics and toxicity (Figure 1). "The major determinant of diarrhea is the amount of SN-38 in the gut lumen, which in turn is determined by the rate of glucuronidation of SN-38 and by the amount of SN-38 transported into the gut," Dr. Ratain said.
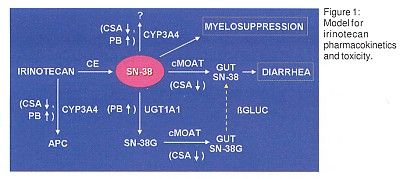
The biliary ratio (SN-38/SN-38G) has been used to validate this model. Dr. Ratain reported that in 26 prospectively studied patients treated with irinotecan, those with higher biliary ratio or biliary index were significantly more likely to have grade 3 or grade 4 diarrhea. "There was a highly significant relationship (P = .003) between biliary index and degree of diarrhea but not between irinotecan dose and diarrhea," Dr. Ratain said.
Human UGT-1 Locus
Researchers used rat models to sort out the factors behind this variability. These studies pointed to the human UGT-1 locus, which encodes multiple forms of UDP-glucuronosyl transferase (UGT). Not only does this locus come in several versions, but frequencies of the UGT1A1 promoter region vary distinctively among ethnic groups.
Dr. Ratain said that in vitro studies have shown that people who have one or two copies of the UGT1A1 7 allele (ie, genotypes 7/7 or 6/7) are less able to glucuronate SN-38 than those whose genotypes are alleles 6/6. "This suggested a relationship between genotype and toxicity in the clinic," Dr. Ratain said.
Examination of genotypes in patients in clinical studies showed that patients either heterozygous or homozygous for the 7 allele are significantly more likely to have grade 2 to 4 diarrhea when treated at 300 mg/m2. Those with 6/7 or 7/7 genotypes are also at greater risk for serious neutropenia at the 300 mg/m2 dose. Studies at the currently recommended dose of 350 mg/m2 dose are ongoing. "The subset of patients who develop diarrhea are likely to be genotypes 7/7 or 6/7," Dr. Ratain said.
Japanese researchers have studied frequencies of the Gly71Arg allele in various populations and in Japanese newborns with hyperbilirubinemia. Dr. Ratain said that these studies show the allele frequency varies from 0 in Germans to 0.23 in Koreans and Chinese and 0.32 in infants with hyperbilirubinemia. "Coding region polymorphisms are very common in Asian populations," he said.
DNA Sequence Diversity
A related area is DNA sequence diversity in genes affecting the activity of CYP3A in the cytochrome P450 system. "Twenty percent of the population has the CYP3A5 allele, and irinotecan is a substrate for CYP3A5," Dr. Ratain said.
The National Institutes of Health Pharmacogenetics Research Network and Knowledge Base is expected to provide some of that information. Dr. Ratain is studying 300 patients in an ongoing Cancer and Leukemia Group B (CALGB) trial of irinotecan/leucovorin who will have DNA specimens collected to enable investigators to look back after study completion and determine whether differences in toxicity correlated with genotype. Other pharmacogenetics studies underway involve drug metabolizing enzymes, asthma treatment, tamoxifen (Nolvadex), membrane transport proteins, as well as minority populations.
How Supportive Care Methods Can Improve Oncology Outcomes
Experts discussed supportive care and why it should be integrated into standard oncology care.