Promising Efficacy for Preoperative Chemoradiotherapy With Capecitabine/ Irinotecan in Locally Advanced Rectal Cancer
MANNHEIM, GERMANY-Complete or nearly complete responsesoccurred in 41% of patients in aphase II trial of capecitabine (Xeloda)
MANNHEIM, GERMANY-Complete or nearly complete responsesoccurred in 41% of patients in aphase II trial of capecitabine (Xeloda)and irinotecan (Camptosar) for neoadjuvanttreatment of patients withlocally advanced rectal cancer (abstract3589). "The efficacy is promising," theinvestigators concluded, and the regimen"is feasible and more convenientthan infusional 5-FU-based chemoradiation."
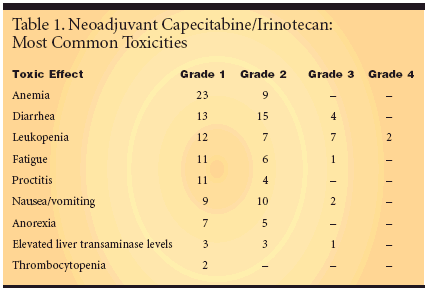
A total of 36 patients were recruitedto receive weekly neoadjuvantirinotecan (50 mg/m2) 1 hour beforeradiotherapy and capecitabine (1,000mg/m2 on days 1 to 38) with a concurrentradiotherapy dose of 50.4 Gy (45+ 5.4 Gy). Dose reductions and/orpostponement of chemotherapy wererequired in 13 patients. Surgery wasscheduled 4 to 6 weeks after completionof chemoradiotherapy.Trial participants had clinical stageT3/4 Nx or N+ rectal cancer. The medianage was 62 years, and themale:female ratio was 27:9. The EasternCooperative Oncology Group(ECOG) performance status was 0 in15 patients, 1 in 18 patients, and 2 in 3patients. The median distance of thetumor from the anal verge was 5 cm.Three patients had local recurrences.Anemia Tops Toxicity ListAccording to updated data reportedby Frank Willeke, MD, of theMannheim University Clinic in Germany,34 patients had resection of theprimary tumor and 41% had completeor nearly complete tumor remission.One patient refused surgery, andone patient was diagnosed with metastasesbefore surgery could be performed.The most common toxicities wereanemia, diarrhea, and leukopenia. Theonly grade 4 toxicities reported weretwo cases of leukopenia (see Table 1on previous page).Postoperative complications includedbowel atonia and bladder dysfunction.Five patients needed surgicalrevision, and three patients hadfistulas. One patient died postoperativelydue to septic complications concerninga perirectal abscess.