Radiolabeled TNT MoAb Effective in Inoperable Lung Cancer
LOS ANGELES-An investigational radiolabeled monoclonal antibody (MoAb) that targets the necrotic core of solid tumors (Cotara, Peregrine Pharmaceuticals Inc., Tustin, California) produced good response rates when injected intratumorally into lung cancers. Alan L. Epstein, MD, PhD, of the University of Southern California Keck School of Medicine, reported the results at the 49th Annual Meeting of the Society of Nuclear Medicine (abstract 1265).
LOS ANGELESAn investigational radiolabeled monoclonal antibody (MoAb) that targets the necrotic core of solid tumors (Cotara, Peregrine Pharmaceuticals Inc., Tustin, California) produced good response rates when injected intratumorally into lung cancers. Alan L. Epstein, MD, PhD, of the University of Southern California Keck School of Medicine, reported the results at the 49th Annual Meeting of the Society of Nuclear Medicine (abstract 1265).
The study, conducted at the Shanghai Tumor Hospital, China, included 43 patients with stage IIIb or IV lung cancer. The average patient age was 57 (range, 31 to 74). All patients had an anticipated survival of at least 3 months. Needle biopsy of the tumors showed that 24 patients had adenocarcinoma, 12 patients had squamous carcinoma, 6 had small-cell lung cancer, and 1 had adeno-squamous carcinoma.
The patients were randomized to receive the Tumor Necrosis Therapy (TNT) agent (131I-chTNT) intravenously (22 patients), intratu-morally (16 patients), or by both methods (5 patients). The dose of 0.8 mCi/kg was repeated 2 weeks later. The intratumoral injection was directed by thoracic CT using a multiple-hole needle. "Using this method, it was possible to use multiple sites of injection in order to assure adequate spread of the antibody throughout the mass of the tumor," Dr. Epstein said.
The best results were obtained in the intratumorally injected group (56% complete and partial responses). The combined IV/intratumoral group had a 40% overall response rate, and the IV administration group, 9%. For all three groups combined, the complete response rate was 4.56%, partial response rate 25.5%, and stable disease 58.1%, for an overall rate of disease control of 88%.
None of the patients developed a HAMA or HACA response. Transient fatigue, appetite loss, and vomiting were reported but did not require treatment. The major adverse event was mild bone marrow toxicity in 20 patients. Only 2 patients had grade 3 neutropenia or thrombocytopenia.
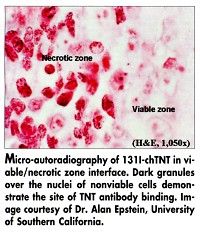
TNT antibodies are directed against intracellular nuclear antigens (DNA/histones) to target degenerating cells present in tumors. Solid tumors, unlike normal tissues, consist of three distinct areas: viable cells, recent areas of necrosis, and old necrotic zones. The TNT antibodies enter the tumor mass and bind specifically to cell ghosts present in both new and old areas of necrosis (see Figure). Normal tissues, which have an active reticuloendothelial system, do not contain degenerating cells for any length of time and are therefore unreactive with TNT antibodies.
"Peregrine is reviewing the intratumoral injection techniques used in the study. This will assist us in designing a lung cancer study in the United States," said Edward Legere, president and CEO of Peregrine Pharmaceuticals.