Radiotherapy for Cutaneous Malignant Melanoma: Rationale and Indications
The use of radiation as adjuvant therapy for patients with cutaneousmalignant melanoma has been hindered by the unsubstantiatedbelief that melanoma cells are radioresistant. An abundance of literaturehas now demonstrated that locoregional relapse of melanoma iscommon after surgery alone when certain clinicopathologic featuresare present. Features associated with a high risk of primary tumor recurrenceinclude desmoplastic subtype, positive microscopic margins,recurrent disease, and thick primary lesions with ulceration or satellitosis.Features associated with a high risk of nodal relapse include extracapsularextension, involvement of four or more lymph nodes, lymphnodes measuring at least 3 cm, cervical lymph node location, and recurrentdisease. Numerous studies support the efficacy of adjuvant irradiationin these clinical situations. Although data in the literatureremain sparse, evidence also indicates that elective irradiation is effectivein eradicating subclinical nodal metastases after removal of theprimary melanoma. Consequently, there may be an opportunity to integrateradiotherapy into the multimodality treatment of patients at highrisk of subclinical nodal disease, particularly those with an involvedsentinel lymph node. Such patients are known to have a low rate ofadditional lymph node involvement, and thus in this group, a shortcourse of radiotherapy may be an adequate substitute for regional lymphnode dissection. This will be the topic of future research.
ABSTRACT: The use of radiation as adjuvant therapy for patients with cutaneous malignant melanoma has been hindered by the unsubstantiated belief that melanoma cells are radioresistant. An abundance of literature has now demonstrated that locoregional relapse of melanoma is common after surgery alone when certain clinicopathologic features are present. Features associated with a high risk of primary tumor recurrence include desmoplastic subtype, positive microscopic margins, recurrent disease, and thick primary lesions with ulceration or satellitosis. Features associated with a high risk of nodal relapse include extracapsular extension, involvement of four or more lymph nodes, lymph nodes measuring at least 3 cm, cervical lymph node location, and recurrent disease. Numerous studies support the efficacy of adjuvant irradiation in these clinical situations. Although data in the literature remain sparse, evidence also indicates that elective irradiation is effective in eradicating subclinical nodal metastases after removal of the primary melanoma. Consequently, there may be an opportunity to integrate radiotherapy into the multimodality treatment of patients at high risk of subclinical nodal disease, particularly those with an involved sentinel lymph node. Such patients are known to have a low rate of additional lymph node involvement, and thus in this group, a short course of radiotherapy may be an adequate substitute for regional lymph node dissection. This will be the topic of future research.
Because of improved public awareness, most cases of cutaneous malignant melanoma are diagnosed at an early stage and can be cured by simple surgical resection. Local recurrence after wide local excision of primary disease is rare, and with few exceptions, the role of adjuvant local therapy in primary melanoma is limited. As the thickness of the primary melanoma increases, however, so does the risk of lymphatic spread. Surgical resection of clinically documented regional disease results in satisfactory regional control, but for certain patients adjuvant regional irradiation is indicated to prevent potentially morbid regional relapse.
For patients without clinically apparent lymphatic involvement, the risk of subclinical nodal disease may be predicted from clinicopathologic features of the primary lesion. In patients with a high risk of subclinical regional disease, approaches have included observation of the nodal basin (with delayed dissection as needed), elective lymph node dissection, and, less frequently, elective lymph node irradiation.
Although elective treatment results in lower rates of nodal recurrence, findings in the four randomized trials investigating the effect of elective dissection on overall survival were negative.[ 1-4] Despite these results, recent developments in sentinel lymph node biopsy techniques have renewed interest in regional lymph node dissection. Patients with an involved sentinel lymph node generally undergo completion dissection. The role of ra diotherapy after sentinel lymph node biopsy remains largely undefined.
In this article we review the rationale and indications for radiotherapy in cutaneous malignant melanoma. We focus first on adjuvant irradiation of the primary tumor site or regional lymph node basins after surgical excision of the primary tumor or dissection of lymph nodes. We then turn to the evolving role of elective nodal irradiation for patients with clinically node-negative disease but a high risk of subclinical nodal involvement. Finally, we discuss the implications of recent developments in sentinel lymph node biopsy for the use of elective lymph node treatment.
Adjuvant Irradiation of the Primary Site
TABLE 1

Local Recurrence Rates After Surgery Alone for Primary Tumor by High-Risk Pathologic Characteristics
Surgical resection remains the primary mode of therapy for cutaneous malignant melanoma. In general, local recurrence after adequate wide local excision of primary tumors is rare, occurring in fewer than 5% of cases. As detailed in Table 1, the rate of local recurrence is higher when primary lesions are thicker than 4 mm, are located on the head or neck, or are associated with ulceration or satellitosis.[2,5-17]
Even when these factors are present, however, the rate of local recurrence is generally less than 15%, although combinations of these factors- such as thickness greater than 4 mm with ulceration or head and neck location-most likely further increase the risk of local recurrence.[ 6,14] Desmoplastic melanoma, a rare variant of cutaneous melanoma, is associated with a clearly increased local recurrence rate.[18-23] These tumors typically present in elderly patients, have a predilection for head and neck sites, and are associated with perineural invasion. Recurrence rates as high as 50% have been reported after ostensibly adequate wide local excision of desmoplastic melanomas (Table 1).
Adjuvant Radiotherapy Data
TABLE 2

Indications for Adjuvant Irradiation in Melanoma Patients
Few series have examined the use of adjuvant irradiation of the primary tumor site in the presence of high-risk clinicopathologic features. The first report of this approach was that of Dickson, who analyzed outcomes in a series of 234 patients according to primary treatment.[24] Treatment consisted of local excision alone (71 patients), radical surgery alone (42 patients), or surgery followed by adjuvant radiotherapy (121 patients). In the adjuvant radiotherapy group, surgery usually involved only simple excision or electrocautery. Radium (surface molds or teleradium therapy) or orthovoltage x-rays were used with almost equal frequency-the latter to deliver a dose of 5,000 cGy in 10 days to the primary tumor site. Although the result in terms of local disease control was not reported, overall survival after simple excision and radio- therapy was similar to that after wide local excision and skin grafting.
Johanson et al reported on a group of 54 patients who underwent surgery and radiotherapy for nodular melanoma to a total dose of 24 Gy in three fractions delivered on days 0, 7, and 21.[25] Of the nine patients who received radiotherapy to the primary tumor site for gross or microscopic residual disease, only one developed progressive local disease.
Although prospective studies are lacking, the available data support a strategy of adjuvant irradiation when local recurrence is of concern. Indications for irradiation of the primary tumor site are detailed in Table 2 and include desmoplastic melanoma, but primary thick primary tumors with associated ulceration or satellitosis, close or positive resection margins where re-resection might compromise cosmesis, and locally recurrent disease are also associated with an increased local failure rate and adjuvant irradiation may be warranted. The primary tumor site may also be irradiated when features of the primary tumor by themselves do not indicate the need for local adjuvant irradiation but regional nodal irradiation is indicated (see below).
Adjuvant Irradiation of the Regional Lymph Nodes
TABLE 3
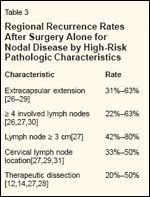
Regional Recurrence Rates After Surgery Alone for Nodal Disease by High-Risk Pathologic Characteristics
For patients who present with documented nodal disease or nodal recurrence after local excision of primary melanoma, therapeutic nodal dissection results in regional control in more than 85% of patients overall. However, several features (Table 3) substantially decrease this satisfactory nodal control rate and indicate a need for adjuvant radiotherapy to avoid uncontrolled regional recurrence. In most series, the presence of any one of these high-risk features results in a 30% to 50% rate of subsequent nodal recurrence after surgery alone.
Although these high-risk features also predict for increased rates of hematogenous metastases, the importance of maintaining regional control and avoiding unmanageable regional recurrence should not be underestimated. The most consistently reported high-risk feature is extracapsular extension, but other factors must also be considered when patients are evaluated for adjuvant therapy.
High-Risk Features
• Extracapsular Extension-In an early surgical series from M. D. Anderson Cancer Center that included 1,001 patients with nodal metastasis, the nodal failure rate after surgery alone was significantly higher when lymph nodes were described as matted (eg, gross extracapsular extension).[ 26] Investigators from Roswell Park Cancer Institute also reported a significantly elevated regional failure rate in the presence of extracapsular extension.[27] In their series of 338 patients, the nodal failure rate was 63% when extracapsular extension was noted microscopically. This association remained significant on multivariate analysis. In another series, from the John Wayne Cancer Institute, extracapsular extension was the single most important predictor of subsequent regional failure after surgery alone.[28] Reported rates of regional failure after surgery alone for nodal metastases with associated extracapsular extension range from 31% to 63% (Table 3).[26-29]
• Number of Involved Nodes-Another factor associated with an increased risk of regional recurrence is a high number of involved lymph nodes. Lee and colleagues reported regional recurrence rates of 25% when 1 to 3 nodes were involved, 46% for 4 to 10 involved nodes, and 63% for more than 10 involved nodes (P = .0001).[27] Two additional series also revealed an increasing risk of regional recurrence with an increasing number of involved lymph nodes.[28,30] Reported rates of regional failure increase rapidly when four or more lymph nodes are involved, ranging from 22% to 63% (Table 3). Although not specifically addressed in most studies, it is reasonable to predict a high correlation between a high number of positive nodes and the presence of extracapsular extension.
• Size of Lymph Nodes-Lymph node size has less frequently been cor- related with the rate of recurrence, but Lee and colleagues did report higher regional recurrence rates with larger lymph nodes: 25% when lymph nodes were smaller than 3 cm, 42% when they were 3 to 6 cm, and 80% when they were larger than 6 cm (P < .001).[27] This finding is not surprising, as the probability of extracapsular extension generally increases with increasing nodal size.
• Clinical Factors-Finally, clinical factors may also predict an increased rate of regional recurrence. At least two series have reported that nodal recurrence rates are higher for cervical nodal disease than for disease in the axillary or inguinal basins.[ 27,31] Also, some series have suggested that the risk of regional recurrence is higher in patients who undergo therapeutic dissection for clinically apparent disease than in patients who have subclinical nodal metastases discovered at the time of elective dissection.
Specifically, in an M. D. Anderson series including patients with cervical lymph node metastases treated with therapeutic modified neck dissection, the regional recurrence rate was 50% for patients with multiple positive lymph nodes as compared with a regional recurrence rate of only 17% when dissection was performed electively but lymph nodes were found to be involved.[12] Other series, including patients with disease in any nodal basin, have reported regional recurrence rates ranging from 20% to 50% when dissection is performed therapeutically (Table 3).[14,27,28]
Adjuvant Radiotherapy Data
TABLE 4
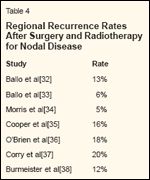
Regional Recurrence Rates After Surgery and Radiotherapy for Nodal Disease
Data from several retrospective series are now available suggesting significant improvements in regional control when adjuvant nodal irradiation is delivered (Table 4).[32-38] In an analysis from M. D. Anderson, the regional recurrence rate in patients treated with adjuvant radiotherapy for axillary nodal metastases was only 13%, despite the fact that the majority of patients presented with one or more of the high-risk features outlined in Table 3.[32] In a separate analysis, we reviewed the records of 160 patients with cervical lymph node metastases and a high risk of regional recurrence and found a 6% 10-year recurrence rate after adjuvant radiotherapy.[33] These findings are consistent with those of other adjuvant radiotherapy series in which regional recurrence rates are consistently below what has been reported after surgery alone despite the presence of adverse clinicopathologic risk factors (compare Tables 3 and 4).
Review of published series, therefore, suggests a clear association between the risk of regional recurrence and the presence of extracapsular ex-tension, the number of involved lymph nodes, size of the lymph nodes, and the site of disease. Although no series has directly analyzed regional recurrence rates according to disease presentation (ie, recurrence after prior lymph node dissection vs primary presentation), recurrent disease is a wellaccepted risk factor for subsequent relapse and indicates the need for radiotherapy. Although therapeutic (vs elective) dissection was associated with an increased risk of nodal recurrence in several retrospective series, at M. D. Anderson we have reserved adjuvant irradiation of lymph node basins for patients who have combinations of therapeutic dissection and other high-risk features. Indications for adjuvant regional radiotherapy are summarized in Table 2.
Elective Irradiation of Regional Lymph Nodes in Patients Without Clinically Evident Nodal Disease
Surgical experience has revealed high rates of subclinical nodal involvement and nodal recurrence after wide local excision of cutaneous malignant melanomas that are clinically nodenegative but greater than 4 mm thick or ulcerated. The rate of lymph node involvement according to primary tumor thickness, documented using sentinel lymph node biopsy techniques, is shown in Table 5. The rate of involvement is generally less than 5% for lesions 0.75 mm or thinner, 10% for lesions 0.76 to 1.50 mm, 20% for lesions 1.51 to 4.0 mm, and 30% to 50% for lesions thicker than 4.0 mm.[39-47] These reported incidences are based on immunohistochemical staining; the use of reverse transcriptase-polymerase chain reaction would increase the detection rate significantly. This assertion is supported by the fact that the documented regional recurrence rate observed after local excision of primary melanoma actually ranges from 20% to 60%.[1,2,4,48-55]
TABLE 5

Risk of Positive Sentinel Lymph Node by Primary Tumor Thickness
Approaches to addressing subclinical regional disease have included observation of the nodal basin, with de- layed dissection as needed; elective lymph node dissection; and, less frequently, elective lymph node irradiation. Although elective treatment results in lower rates of nodal recurrence, the four completed randomized trials did not show a benefit of elective dissection in terms of overall survival.[1-4]
A similar lack of effect on overall survival would be expected from elective regional irradiation, but studies have now shown that elective irradiation can in fact effectively control subclinical regional disease and obviate dissection. This option is particularly attractive for patients in whom the disease status of the lymph nodes will have no bearing on subsequent therapy. The most obvious candidates are elderly patients and those with considerable comorbid conditions, but any patient who is not a candidate for systemic therapy protocols or dissection of the regional lymphatics may benefit from this approach. At M. D. Anderson, patients with clinically node-negative cutaneous melanoma of the head and neck with a thickness of at least 1.5 mm or Clark level of at least IV have routinely been offered elective irradiation.
Elective Radiotherapy Data
In 157 patients with stage I or II cutaneous melanoma of the head and neck, the policy of elective regional radiotherapy after wide local excision of the primary lesion resulted in an actuarial regional control rate of 89% at both 5 and 10 years.[56] This regional control rate was significantly better than the rate expected on the basis of the Breslow thicknesses in these patients.
TABLE 6

Indications for Elective Nodal Irradiation
Indications for elective nodal irradiation used at M. D. Anderson are outlined in Table 6. To our knowledge, no data regarding elective irradiation of other nodal basins are available, but this is certainly a reasonable therapeutic option for some patients.
Radiation Dose Schedule and Treatment Volumes
The most appropriate radiation dose schedule for patients with cutaneous malignant melanoma remains somewhat controversial. The Radiation Therapy Oncology Group (RTOG) launched a phase III trial in 1983 to compare the effects of four fractions of 8 Gy given at weekly intervals with those of 20 fractions of 2.5 Gy given daily, 5 days per week. A total of 137 patients were entered in this study, which included all sites of metastasis other than the abdomen or brain. Most patients had soft-tissue, cutaneous, or nodal disease. Approximately half of the lesions in this trial were greater than 5 cm in largest diameter. There was no difference in complete or partial response rates according to fraction size: The complete response rate was 24.2% for four fractions and 23.4% for 20 fractions, and the partial response rate was 35.5% for four fractions and 34.4% for 20 fractions.[ 57] Unfortunately, the duration of response-an important indicator of the magnitude of cell killing-was not reported.
Although neither of the two treatments was superior to the other, both were certainly effective in terms of clinical response, refuting the alleged radioresistance of malignant melanoma.
Specific Recommendations
We have continued to advocate a hypofractionated regimen because of the positive experiences at M. D. Anderson with this approach, a continued belief that some melanoma cell lines are more sensitive to large doses per fraction, and patient convenience.[ 32,33,56,58,59] For most clinical situations, we recommend 30 Gy in five 6-Gy fractions delivered over 2.5 weeks (on Mondays and Thursdays, or Tuesdays and Fridays). When irradiation of the brain, brainstem, or spinal cord is required, the dose must not exceed 24 Gy, or a more conventional fractionation schedule may be used.
For adjuvant irradiation of the primary tumor site, the width of the portal will depend on the proximity of critical structures, but generally 2-to 4-cm margins using electrons with appropriately thick bolus material are sufficient.
FIGURE 1
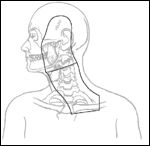
Portals for Irradiation of Cutaneous Head and Neck Melanoma
For nodal irradiation, portals should encompass the predicted draining lymphatics with adequate shielding of nearby critical structures. When the cervical lymph nodes are irradiated, the patient may be immobilized in an "open neck" position (Figure 1). Computed tomography planning is used to choose electron energies, and junctions are shifted twice during the course of irradiation. The dose is prescribed to Dmax. Tissue-equivalent bolus may be used to limit the dose to the temporal lobe and the larynx. Portals generally encompass the site of the primary lesion, the superficial parotid gland, and the entire ipsilateral neck, including the supraclavicular fossa. The primary lesion may be excluded if the indication for irradiation is nodal recurrence 1 year or more after treatment of a primary melanoma.
FIGURE 2
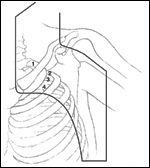
Portal for Irradiation of Axillary Nodal Metastases
For the axilla, anterior and posterior photon beams are used to cover the ipsilateral low cervical, supraclavicular, and level I, II, and III axillary lymph node regions (Figure 2). Either wedge or compensation filters are used to correct for dose inhomogeneities caused by surface irregularities through the field of irradiation. Dose heterogeneity may also be avoided by prescribing to a volume and, if necessary, allowing the isocenter dose to be 3% to 6% below the prescribed dose.
For inguinal lymph node metastases, radiation portals are generally less comprehensive than for the cervical and axillary basins. In an attempt to minimize the risk of long-term lymphedema, radiation is directed to documented clinical disease only, with no attempt to cover potential subclinical extension to the external or common iliac lymph node chains unless initially involved. Also, it is believed that 30 Gy in five fractions exceeds the tolerance of the small bowel, and thus every attempt should be made to limit the small bowel dose to less than 24 Gy using appropriate technique and shielding. If this is not possible, more conventional fractionation should be used.
Toxicity
In general, nodal dissection is well tolerated and adjuvant irradiation only modestly increases the overall morbidity of the procedure. In one study, after cervical lymphadenectomy, 7% of patients had functional deficits and 6% experienced long-term pain.[60] Using actuarial techniques, we reported 5-year complication rates of 12% for grade 1 and 10% for grade 2 complications.[ 33] This series included 160 patients who had therapeutic neck dissection and adjuvant radiotherapy for lymph node metastases. The grade 1 complications all involved the irradiated skin or mucous membranes and corresponded to an RTOG/European Organization for Research and Treatment of Cancer late radiation morbidity score of grade 1, which is defined as slight induration, loss of subcutaneous fat, or slight atrophy. The grade 2 complications included nine cases in which conservative medical treatment was required for decreased hearing (three patients), clinical hypothyroidism (two patients), wound breakdown (two patients), bone exposure (one patient), or mild ear pain (one patient).
After axillary dissection, Urist and colleagues reported edema, functional deficits, and pain in 1%, 9%, and 6% of patients, respectively.[60] In a separate series reviewing regional node dissection for axillary melanoma, the reported risk of long-term lymphedema was only 3%.[31] In our series of 89 patients who had undergone axillary lymph node dissection and adjuvant hypofractionated radiotherapy at M. D. Anderson, we found late arm edema in 26 patients.[32] The 5-year actuarial rates of edema by severity were 21% for grade 1 (transient or asymptomatic), 19% for grade 2 (requiring medical management), and 1% for grade 3 (requiring surgical therapy). In an Australian series, the rate of upper-extremity edema after nodal dissection and hypofractionated radiotherapy was 10% at 20 months.[61]
That these rates are higher than those reported in series examining lymphadenectomy alone may reflect patient selection factors, with adjuvant radiotherapy being delivered to patients with more regionally advanced disease and requiring more extensive surgical dissection. Also, the incidence of mild to moderate arm edema seen in this setting at M. D. Anderson is similar to what is seen after nodal dissection and comprehensive axillary irradiation for locally advanced breast cancer.
The risk of clinically significant lower-extremity edema after inguinal lymph node dissection depends on the extent of dissection and the definition of edema but ranges from 10% to 28%.[60,62-64] Series specifically reporting the rate of lymphedema according to severity have indicated that long-term lymphedema requiring medical management occurs in 10% to 19% of patients.[62,63] In a recent Australian series, the rate of moderate lymphedema requiring medical management after groin dissection and adjuvant irradiation was 48% at 4 years.[61] Again, it was unclear whether this rate of lymphedema was due solely to the adjuvant irradiation or if the extent of dissection and burden of disease may have played a role.
In general, patients will have atrophy and loss of subcutaneous fat after nodal irradiation. Mild to moderate long-term lymphedema requiring medical management (physical therapy and compressive devices) occurs in a minority of patients and is seen most commonly after adjuvant irradiation for inguinal nodal metastases. Despite the documented risk of long-term lymphedema, in appropriately selected patients the risk of major complications is far outweighed by improvements in regional control and avoidance of uncontrolled regional recurrence.
Irradiation of Regional Lymph Nodes in Patients With a Positive Sentinel Lymph Node Biopsy
Although data from the literature suggest that adjuvant irradiation after nodal dissection decreases the rate of local recurrence, this has not been proven. Similarly, evidence in favor of elective irradiation has been limited to a single institution and suffers from the same limitations as elective lymph node dissection.
Wider availability of sentinel lymph node biopsy now provides an opportunity to examine the potential benefits of elective nodal treatment in a more appropriately selected patient population. It has been suggested that lymph node dissection might benefit only those patients who are known to have nodal involvement. Specifically, Cascinelli and colleagues reported a worse survival rate in patients undergoing delayed dissection for regional recurrence than in patients undergoing elective lymph node dissection and found to have nodal disease.[2]
By identifying early nodal metastases, sentinel lymph node biopsy allows proponents of elective lymph node dissection to perform dissection in patients with documented nodal disease. As a corollary, sentinel lymph node biopsy might also allow proponents of elective regional irradiation to irradiate the regional lymphatics in lieu of completion dissection after sentinel node mapping. This policy would provide prognostic information and allow appropriate stratification of patients according to risk factors for clinical trials examining the role of adjuvant systemic therapy, while at the same time providing regional control and eliminating the possibility of morbidity from surgical dissection.
TABLE 7
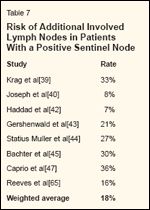
Risk of Additional Involved Lymph Nodes in Patients With a Positive Sentinel Node
Evidence supporting the validity of this proposed treatment is twofold. First, series examining adjuvant radiotherapy for patients with high-risk clinicopathologic features noted at the time of therapeutic dissection for nodal disease indicate that radiotherapy is effective at sterilizing microscopic deposits of melanoma cells (Table 4).[32-38] Second, as shown in Table 7, the likelihood of additional nodal disease in patients with a positive sentinel lymph node is approximately 20%, and this disease is usually of minimal microscopic burden.[ 39,40,42-45,47,65] It is likely that radiation can effectively sterilize this burden of microscopic disease, as it does when patients are treated therapeutically. We believe a randomized trial comparing completion lymphadenectomy to therapeutic irradiation for patients with a positive sentinel lymph node is justified.
Conclusions
A growing body of literature supports the efficacy of adjuvant and elective irradiation in patients with cutaneous malignant melanoma and highrisk clinicopathologic features. The effect of irradiation on overall survival remains largely unknown, but the avoidance of locoregional recurrence is clearly beneficial. Future research should focus on the integration of radiotherapy into systemic therapy protocols. Despite the abundance of retrospective data, only well-designed randomized trials can establish radiotherapy as an appropriate component of multidisciplinary treatment for patients with malignant melanoma. Such trials are urgently needed.
Financial Disclosure: The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
Supported in part by grant CA06294 from the National Cancer Institute and by the Gilbert H. Fletcher Chair.
References:
1.
Veronesi U, Adamus J, Bandiera DC, etal: Delayed regional lymph node dissection instage I melanoma of the skin of the lower extremities.Cancer 49:2420-2430, 1982.
2.
Cascinelli N, Morabito A, Santinami M,et al: Immediate or delayed dissection of regionalnodes in patients with melanoma of thetrunk: A randomised trial. Lancet 351:793-796,1998.
3.
Balch CM, Soong S Jr, Ross MI, et al:Long-term results of a multi-institutional randomizedtrial comparing prognostic factors andsurgical results for intermediate thickness melanomas(1.0 to 4.0 mm). Intergroup MelanomaSurgical Trial. Ann Surg Oncol 7:87-97, 2000.
4.
Sim FH, Taylor WF, Pritchard DJ, et al:Lymphadenectomy in the management ofstage I malignant melanoma: A prospectiverandomized study. Mayo Clin Proc 61:697-705, 1986.
5.
Urist MM, Balch CM, Soong S, et al: Theinfluence of surgical margins and prognosticfactors predicting the risk of local recurrencein 3445 patients with primary cutaneous melanoma.Cancer 55:1398-1402, 1985.
6.
Ames FC, Balch CM, Reintgen D: Localrecurrence and their management, in Balch CM,Houghton AN, Milton GW, Sober AJ, Soong S,(eds): Cutaneous melanoma, pp 287-294. Philadelphia,J.B Lippincott Company, 1992.
7.
Roses DF, Harris MN, Rigel D, et al: Localand in-transit metastases following definitiveexcision for primary cutaneous malignantmelanoma. Ann Surg 198:65-69, 1983.
8.
Karakousis CP, Balch CM, Urist MM, etal: Local recurrence in malignant melanoma:Long-term results of the multiinstitutional randomizedsurgical trial. Ann Surg Oncol 3:446-452, 1996.
9.
Ng AK, Jones WO, Shaw JH: Analysis oflocal recurrence and optimizing excision marginsfor cutaneous melanoma. Br J Surg88:137-142, 2001.
10.
Heaton KM, Sussman JJ, GershenwaldJE, et al: Surgical margins and prognostic factorsin patients with thick (>4mm) primarymelanoma. Ann Surg Oncol 5:322-328, 1998.
11.
Neades GT, Orr DJ, Hughes LE, et al:Safe margins in the excision of primary cutaneousmelanoma. Br J Surg 80:731-733, 1993.
12.
Byers RM: The role of modified neckdissection in the treatment of cutaneous melanomaof the head and neck. Arch Surg121:1338-1341, 1986.
13.
Loree TR, Spiro RH: Cutaneous melanomaof the head and neck. Am J Surg 158:388-391, 1989.
14.
O’Brien CJ, Coates AS, Petersen-Schaefer K, et al: Experience with 998 cutaneousmelanomas of the head and neck over 30years. Am J Surg 162:310-314, 1991.
15.
Fisher SR, O’Brien CJ: Head and neckmelanoma, in Balch CM, Houghton AN, SoberAJ, Soong S, (eds): Cutaneous Melanoma,pp 163-174. St Louis, Quality Medical PublishingInc, 1998.
16.
Kelly JW, Sagabiel RW, Calderon W, etal: The frequency of local recurrence andmicrosatellites as a guide to reexcision marginsfor cutaneous malignant melanoma. AnnSurg 200:759-763, 1984.
17.
Leon P, Daly JM, Synnestvedt M, et al:The prognostic implications of microscopicsatellites in patients with clinical stage I melanoma.Arch Surg 126:1461-1468, 1991.
18.
Egbert B, Kempson R, Sagebiel R: Desmoplasticmalignant melanoma: A clinicohistopathologicstudy of 25 cases. Cancer62:2033-2041, 1988.
19.
Beenken S, Byers R, Smith JL, et al:Desmoplastic melanoma. Arch OtolaryngolHead Neck Surg 115:374-379, 1989.
20.
Smithers BM, McLeod GR, Little JH:Desmoplastic melanoma: Patterns of recurrence.World J Surg 16:186-190, 1992.
21.
Calson JA, Dickersin GR, Sober AJ, etal: Desmoplastic neurotropic melanoma: Aclinicopathologic analysis of 28 cases. Cancer75:478-494, 1995.
22.
Quinn MJ, Crotty KA, Thompson JF, etal: Desmoplastic and desmoplastic neurotropicmelanoma: Experience with 280 patients.Cancer 83:1128-1135, 1998.
23.
Payne WG, Kearney R, Wells K, et al:Desmoplastic melanoma. Am Surg 67:1004-1006, 2001.
24.
Dickson RJ: Malignant melanoma: acombined surgical and radiotherapeutic approach.Am J Roentgenol 79:1063-1070, 1958.
25.
Johanson CR, Harwood AR, CummingsBJ, Quirt I: 0-7-21 radiotherapy in nodularmelanoma. Cancer 51:226-232, 1983.
26.
Calabro A, Singletary SE, Balch CM:Patterns of relapse in 1001 consecutive patientswith melanoma nodal metastases. Arch Surg124:1051-1055, 1989.
27.
Lee RJ, Gibbs JF, Proulx GM, et al:Nodal basin recurrence following lymph nodedissection for melanoma: Implications for adjuvantradiotherapy. Int J Radiat Oncol BiolPhys 46:467-474, 2000.
28.
Shen P, Wanek LA, Morton DL: Is adjuvantradiotherapy necessary after positivelymph node dissection in head and neck melanomas?Ann Surg Oncol 7:554-559, 2000.
29.
Monsour PD, Sause WT, Avent JM, etal: Local control following therapeutic nodaldissection for melanoma. J Surg Oncol 54:18-22, 1993.
30.
Miller EJ, Synnestvedt M, Schultz D,et al: Loco-regional nodal relapse in melanoma.Surg Oncol 1:333-340, 1992.
31.
Bowsher WG, Taylor BA, Hughes LE:Morbidity, mortality and local recurrence followingregional node dissection for melanoma.Br J Surg 73:906-908, 1986.
32.
Ballo MT, Strom EA, Zagars GK, et al:Adjuvant irradiation for axillary metastasesfrom malignant melanoma. Int J Radiat OncolBiol Phys 52:964-972, 2002.
33.
Ballo MT, Bonnen MD, Garden AS, etal: Adjuvant irradiation for cervical lymph nodemetastases from melanoma. Cancer 97:1789-1796, 2003.
34.
Morris KT, Marquez CM, Holland JM,et al: Prevention of local recurrence after surgicaldebulking of nodal and subcutaneousmelanoma deposits by hypofractionated radiation.Ann Surg Oncol 7:680-684, 2000.
35.
Cooper JS, Chang WS, Oratz R, et al:Elective radiation therapy for high-risk malignantmelanoma. Cancer J 7:498-502, 2001.
36.
O’Brien CJ, Petersen-Schaefer K,Stevens GN, et al: Adjuvant radiotherapy followingneck dissection and parotidectomy formetastatic malignant melanoma. Head Neck19:589-594, 1997.
37.
Corry J, Smith JG, Bishop M, et al:Nodal radiation therapy for metastatic melanoma.Int J Radiat Oncol Biol Phys 44:1065-1069, 1999.
38.
Burmeister BH, Smithers BM, PoulsenM, et al: Radiation therapy for nodal diseasein malignant melanoma. World J Surg 19:369-371, 1995.
39.
Krag DN, Meijer SJ, Weaver DL, et al:Minimal-access surgery for staging of malignantmelanoma. Arch Surg 130:654-658,1995.
40.
Joseph E, Brobeil A, Glass F, et al: Resultsof complete lymph node dissection in 83melanoma patients with positive sentinel nodes.Ann Surg Oncol 5:119-125, 1998.
41.
Mraz-Gernhard S, Sagebiel RW,Kashani-Sabet M, et al: Prediction of sentinellymph node micrometastasis by histologicalfeatures in primary cutaneous malignantmelanoma. Arch Dermatol 134:983-987,1998.
42.
Haddad FF, Stall A, Messina J, et al: Theprogression of melanoma nodal metastasis isdependent on tumor thickness of the primarylesion. Ann Surg Oncol 6:144-149, 1999.
43.
Gershenwald JE, Thompson W,Mansfield PF, et al: Multi-institutional melanomalymphatic mapping experience: the prognosticvalue of sentinel lymph node status in612 stage I and II melanoma patients. J ClinOncol 17:976-983, 1999.
44.
Statius Muller MG, Borgstein PJ, PijpersR, et al: Reliability of the sentinel node procedurein melanoma patients: analysis of failuresafter long-term follow-up. Ann Surg Oncol7:461-468, 2000.
45.
Bachter D, Michl C, Büchels H, et al:The predictive value of the sentinel lymph nodein malignant melanomas. Recent Results CancerRes 158:129-136, 2001.
46.
Blumenthal R, Banic A, Brand CU, etal: Morbidity and outcome after sentinel lymphnode dissection in patients with early-stagemalignant cutaneous melanoma. Swiss Surg8:209-214, 2002.
47.
Caprio MG, Carbone G, Bracigliano A,et al: Sentinel lymph node detection bylymphoscintigraphy in malignant melanoma.Tumori 88:S43-S45, 2002.
48.
Eldh J, Suurkula M, Holmstrom H: Prognosisfor localized cutaneous melanoma treatedwith wide excision only, with special referenceto development of regional node metastases.Tumori 73:51-54, 1987.
49.
Balch CM: Surgical management of regionallymph nodes in cutaneous melanoma. JAm Acad Dermatol 3:511-524, 1980.
50.
Slingluff CL, Stidham KR, Ricci WM,et al: Surgical management of regional lymphnodes in patients with melanoma. Experiencewith 4682 patients. Ann Surg 219:120-130,1994.
51.
Harrist TJ, Rigel DS, Day CL Jr, et al:“Microscopic satellites” are more highly associatedwith regional lymph node metastasesthan is primary melanoma thickness. Cancer53:2183-2187, 1984.
52.
Carmichael VE, Robins RE, Wilson KS:Elective and therapeutic regional lymph nodedissection for cutaneous malignant melanoma:Experience of the British Columbia CancerAgency, 1972 to 1981. Can J Surg 35:600-604,1992.
53.
Weissmann A, Roses DF, Harris MN, etal: Prediction of lymph node metastases fromthe histologic features of primary cutaneousmalignant melanomas. Am J Dermatopathol6(Suppl):35-41, 1984.
54.
Fisher SR: Elective, therapeutic, anddelayed lymph node dissection for malignantmelanoma of the head and neck: Analysis of1444 patients from 1970 to 1998. Laryngoscope112:99-110, 2002.
55.
Fortner JG, Woodruff J, Schottenfeld D,et al: Biostatistical basis of elective node dissection for malignant melanoma. Ann Surg186:101-103, 1977.
56.
Bonnen MD, Ballo MT, Myers JN, et al:Elective radiotherapy provides regional controlfor patients with cutaneous melanoma of thehead and neck. Cancer. In press.
57.
Sause WT, Cooper JS, Rush S, et al: Fractionsize in external beam radiation therapy inthe treatment of melanoma. Int J Radiat OncolBiol Phys 20:429-432, 1991.
58.
Ang KK, Peters LJ, Weber RS, et al:Postoperative radiotherapy for cutaneous melanomaof the head and neck region. Int J RadiatOncol Biol Phys 30:795-798, 1994.
59.
Ballo MT, Ang KK: Radiation therapyfor malignant melanoma. Surg Clin North Am83:323-342, 2003.
60.
Urist MM, Maddox WA, Kennedy JE,et al: Patient risk factors and surgical morbidityafter regional lymphadenectomy in 204melanoma patients. Cancer 51:2152-2156,1983.
61.
Burmeister BH, Smithers BM, Davis S,et al: Radiation therapy following nodal surgeryfor melanoma: An analysis of late toxicity.ANZ J Surg 72:344-348, 2002.
62.
Hughes TM, A’Hern RP, Thomas JM:Prognosis and surgical management of patientswith palpable inguinal lymph node metastasesfrom melanoma. Br J Surg 87:892-901, 2000.
63.
Karakousis CP, Driscoll DL, Rose B, etal: Groin dissection in malignant melanoma.Ann Surg Oncol 1:271-277, 1994.
64.
Kissin MW, Simpson DA, Easton D, etal: Prognostic factors related to survival andgroin recurrence following therapeutic lymphnode dissection for lower limb malignant melanoma.Br J Surg 74:1023-1026, 1987.
65.
Reeves ME, Delgado R, Busam KJ, etal: Prediction of nonsentinel lymph node statusin melanoma. Ann Surg Oncol 10:27-31,412003.