Recap: Improving Outcomes in Newly Diagnosed Multiple Myeloma
ONCOLOGY® recaps an Around the Practice program moderated by Sagar Lonial, MD, with additional panelists Morie A. Gertz, MD; Parameswaran Hari, MD, MS; and Andrzej Jakubowiak, MD, PhD.
Treating patients with newly diagnosed multiple myeloma presents challenges ranging from choosing optimal therapy regimens to determining transplant eligibility. At an Around the Practice presentation hosted by CancerNetwork®, experts discussed improving outcomes for this patient group using a patient case and audience polling questions. The panel was led by Sagar Lonial, MD, professor and chair of hematology and medical oncology at the Emory University School of Medicine in Atlanta, Georgia.
Transplant-Eligible Newly Diagnosed Multiple Myeloma
To start the discussion, the panelists discussed a case of a man, aged 47 years, whose disease was deemed eligible for transplant.
CASE

Morie A. Gertz, MD, chair of general internal medicine at the Mayo Clinic in Rochester, Minnesota, described his institute’s rationale for deciding transplant eligibility in a patient such as this. One issue, said Gertz, is related to the risk of therapy-related mortality.
“The transplant is not successful if you have therapy-related mortality or [the patient has] 2 months in the hospital,” Gertz said. According to results of a study published in the European Journal of Haematology by Mohamad Mohty, MD, PhD, and colleagues, patients with multiple myeloma who die during early treatment usually do not die from disease progression.1 Gertz said that when determining treatment, patients should be looking at less than 1% risk of mortality, determined by fitness, questionnaires, and scales. However, age is not necessarily a deterrent for transplants, in his opinion. “We transplant [in patients aged] up to 78 [years] with full-dose melphalan at 200 mg/m2,” Gertz noted.
The other myeloma panelists agreed. Parameswaran Hari, MD, MS, who was chief of hematology and oncology at the Medical College of Wisconsin in Milwaukee at the time of filming, added that transplant teams are well placed and skilled at selecting transplant-eligible patients, so oncologists should refer patients they feel are candidates and let that team make the final decision. Andrzej Jakubowiak, MD, PhD, director of the myeloma program at the University of Chicago in Illinois, said that at his institution there are “layers” for selecting patients older than 65 years for transplant, which involves many players on a multidisciplinary team.
Lonial then referred to a paper published by Hari and colleagues utilizing data from the Center for International Blood and Marrow Transplant Research in which 360 patients with multiple myeloma who were 75 years or older underwent autologous hematopoietic stem cell transplantation. The results showed that 100-day transplant mortality was 1%; further, at the 2-year time point, the relapse rate was 27%, the progression-free survival (PFS) rate was 66%, and the overall survival rate was 83%, showing that transplant is feasible in this population.2
Deciding on Induction Therapy
For patients who are transplant eligible, decisions must first be made regarding induction therapy. Jakubowiak stated he would treat this patient with daratumumab (Darzalex) plus carfilzomib (Kyprolis) and dexamethasone (Dara-Kd), although there could be some limitations.
Given the patient’s young age, it is important to select a regimen with the best chance of achieving a durable extended remission, Jakubowiak said. “From that perspective, we have recently seen very good results with quadruplet,” he noted. Daratumumab can be added to backbones of bortezomib (Velcade), lenalidomide (Revlimid), and low-dose dexamethasone (VRd) or carfilzomib, lenalidomide, and dexamethasone (KRd). He then pointed to a 2021 study by C. Ola Landgren, MD, PhD, and colleagues published in JAMA Oncology regarding daratumumab plus carfilzomib, lenalidomide, and dexamethasone (Dara-KRd), which led to high rates of minimal residual disease (MRD) and PFS among patients with multiple myeloma.3
The ongoing phase 2 MASTER trial (NCT03224507), which is examining Dara-KRd, has also demonstrated high response rates in this patient group. Recently reported data at the 63rd American Society of Hematology Annual Meeting & Exposition showed that MRD could assist in therapy selection and inform clinicians about the duration of daratumumab therapy.4
Jakubowiak did warn, however, that adaptations may be necessary for certain patients, particularly older ones. For example, high levels of M protein could indicate renal insufficiency. “It’s okay to go directly to Dara-KRd, in my opinion,” said Jakubowiak, “but maybe with a high risk of complications with the first cycle.” These events could call for a short course of steroids, especially if hypercalcemia is present.
In treating a patient such as this one, Gertz said he would certainly use a 4-drug regimen, for which he favors daratumumab plus VRd (Dara-VRd). “This patient has the best possible prognosis that you could see. I would certainly use a quadruplet [and] I’m going to save carfilzomib for second-line therapy,” explained Gertz.
Deciding When to Use Quadruplet Regimens
Lonial asked the panelists about data they look to when deciding on using a quadruplet regimen over a triplet.
Triplet therapy for multiple myeloma seems to be losing favor as a first-choice treatment, even for very frail patients, according to Gertz. He stated that results of many studies show that patients who achieve MRD negativity will do better. In his opinion, looking at the quadruplet studies, the MRD-negative rates are higher than those reported with triplets.
Hari looked to the GRIFFIN trial (NCT02874742) of Dara-VRd vs VRd alone, for which the primary end point was the rate of stringent complete response (sCR) after autologous stem cell transplantation. Secondary end points included the MRD negativity rate, which was higher in the Dara-VRd group than in those treated with the VRd triplet (P <.0001).5
Jakubowiak added that while he does test for MRD, he does not use it as a guide for therapy. He prefers to use modified quadruplets when necessary, by significantly reducing the dose and scale of dexamethasone and bortezomib, but still exposing patients to 4 drugs. Jakubowiak called this approach “quadruple light.”
Hari reminded the group that evidence from the CASSIOPEIA trial (NCT02541383) also showed that quadruple therapy with VTd was superior to the same triplet without daratumumab. In updated results published in The Lancet Oncology in 2021, daratumumab maintenance in patients with transplant-eligible newly diagnosed multiple myeloma led to significantly longer PFS vs observation.6 With follow-up, PFS benefit with daratumumab appeared only in patients treated with VTd as induction and consolidation.
Determining Adequate Response
In an audience poll, Lonial asked what the participants considered to be an adequate, successful treatment response following induction (Poll question 1).
Poll questions 1 & 2
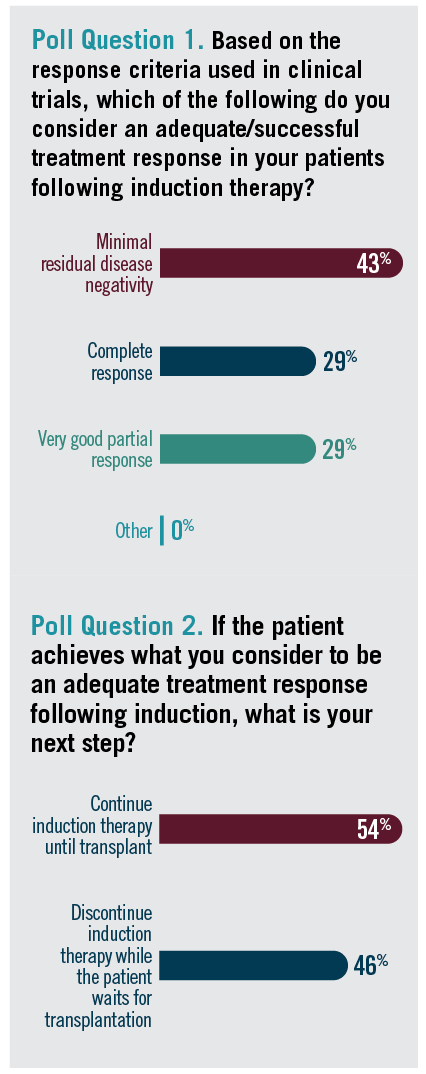
Gertz said it’s hard to answer this question without details about the specific patient being treated. He said that the question “doesn’t take into account what their underlying biology is, whether they have high proliferative rates or adverse genetics. If [they achieve] MRD negativity, I’m cheering for them and waving flags. But if they don’t, I’m not quick to say they have failed treatment. I’m very excited when they’re MRD negative, but I’m not making the treatment decision and going to second-line therapy because I thought it wasn’t deep enough. It depends on their biology.”
Hari stated that he is a big believer in response-adapted therapy, usually starting treatment with Dara-VRd. Most of the patients are in a partial response (PR) or sometimes very good PR (VGPR) by about 8 weeks, he said. If that does not work, he likes to upgrade treatment to Dara-KRd. He tries to get his patients close to VGPR before stem cell collection. “The closer you are to MRD negativity, especially [in] the younger patients, the better the outcomes you get with transplant,” Hari pointed out.
Lonial then asked the audience what they prefer to do once a patient achieves an adequate response following induction therapy (Poll question 2).
Jakubowiak explained that once patients achieve a VGPR or better, he discontinues most treatment and then plans additional posttransplant therapy. “In general, I don’t plan for a deferred transplant even in good responders with a complete response. We consolidate with transplant, then plan to extend the treatment with the initial regimen,” he said. Jakubowiak pointed out that this is in line with the GRIFFIN, MASTER, and CASSIOPEIA studies.
Hari noted that this question is especially important in the case of patients who are handed over from treatment in the community setting to transplant centers. If the referral was made at the second cycle, “there is usually very little gap between finishing induction and going straight to the transplant,” he said. He would be hesitant to discontinue all induction therapy and wait for the transplant, he added; in any case, the wait should not be longer than 2 to 3 weeks.
Jakubowiak agreed that such a break is not advised, but he believes it sometimes happens in the community setting when patients are sent to transplant centers. A short break of 2 to 3 weeks probably doesn’t hurt, he said, but for high-risk patients, a longer wait could be dangerous.
Gertz made the panel responses unanimous; he said prefers early transplantation, with chemotherapy discontinued 14 days prior to the transplant.
Consolidation Therapy
Moving to consolidation vs maintenance therapies, Lonial said that a variety of studies exist touting the benefits of both options. All 3 panelists leaned toward moving straight to maintenance following transplants.
In Gertz’s practice, at day 100, patients are put on a clinical trial or maintenance therapy without consolidation.
Hari said he does not use consolidation, because little evidence of benefit exists. He was, for a time, administering consolidation cycles as seen in the GRIFFIN trial, but he has dropped the daratumumab.
Jakubowiak mentioned the phase 3 STaMINA trial (NCT02322320), which evaluated PFS with the use of lenalidomide maintenance in patients who were given melphalan followed by transplant. “The single-agent lenalidomide was not worse than other strategies,”
Jakubowiak said.
Transplant-Ineligible Newly Diagnosed Multiple Myeloma
Next in the discussion, the panelists reviewed their thoughts about first-line induction therapy for newly diagnosed patients not eligible for transplant. For the purposes of this discussion, they focused on a patient with standard-risk disease (Poll question 3).
Poll questions 3 & 4

Despite the audience favoring triplets of Dara-Rd and VRd, Jakubowiak said
triplet therapy no longer seems to be a go-to treatment, even for frail patients with multiple myeloma, reiterating the superiority of quadruplet therapies. He believes that this would also apply to transplant-ineligible patients. “In this context, I would be favoring Dara-VRd,” he said.
However, Lonial pointed out that PFS data in this population extending to 60 months are not yet available. Hari mentioned that he would use Dara-VRd in patients who are at higher risk. “I would argue that a protease inhibitor is probably tempting for some of these high-risk patients,” Hari stated. “There are no randomized situations that guide us here, but that’s what I do for the 15% to 20% of patients with high-risk disease who are transplant ineligible.” Quadruplet therapy is also how Gertz would approach treatment.
Lonial then asked the panel what would they consider for a relapsed patient, if they were to use a triplet. Most favored daratumumab plus Rd (Dara-Rd), efficacy of which was demonstrated in the phase 3 MAIA trial (NCT02252172) vs Rd alone.7
“If not quadruplet, I would probably go with Dara-Rd as my next choice and would not risk neuropathy in these patients,” added Jakubowiak.
Pivoting to MRD, the panel discussed how these results inform treatment selection in a frail population. Jakubowiak stated that achievement of MRD might not be the end goal in these patients, in whom pushing therapy can do more harm than good.
In patients overall, Jakubowiak said, “We are entering a phase in which we will be making decisions based on MRD. There are ongoing studies in which patients who are in longstanding remission [with] sustained MRD activity are considered for discontinuation.”
Lonial then polled the viewers about how they determined treatment duration for transplant-ineligible patients (Poll question 4).
While Hari agreed with the decision of the majority of respondents to treat until progression, he pointed out that patients in the real world often do stop therapy for one reason or another.
References
1. Mohty M, Cavo M, Fink L, et al. Understanding mortality in multiple myeloma: findings of a European retrospective chart review. Eur J Haematol. 2019;103(2):107-115. doi:10.1111/ejh.13264
2. Munshi PN, Vesole DH, St Martin A, et al. Outcomes of upfront autologous hematopoietic cell transplantation in patients with multiple myeloma who are 75 years old or older. Cancer. 2021;127(22):4233-4239. doi:10.1002/cncr.33831
3. Landgren O, Hultcrantz M, Diamond B, et al. Safety and effectiveness of weekly carfilzomib, lenalidomide, dexamethasone, and daratumumab combination therapy for patients with newly diagnosed multiple myeloma: the MANHATTAN nonrandomized clinical trial. JAMA Oncol. 2021;7(6):862-868. doi:10.1001/jamaoncol.2021.0611
4. Costa LJ, Chhabra S, Callander NS, et al. Daratumumab, carfilzomib, lenalidomide and dexamethasone (Dara-KRd), autologous transplantation and MRD response-adapted consolidation and treatment cessation. final primary endpoint analysis of the MASTER trial. Blood. 2021;138(suppl 1):481. doi:10.1182/blood-2021-145494
5. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/blood.2020005288
6. Moreau P, Hulin C, Perrot A, et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(10):1378-1390. doi:10.1016/S1470-2045(21)00428-9
7. Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582-1596. doi:10.1016/S1470-2045(21)00466-6
Recap: Recent Advances in the Treatment of Metastatic Castration-Sensitive Prostate Cancer
September 18th 2022Expert oncologists review key studies in the metastatic castration-resistant prostate cancer treatment landscape and discuss how evidence can be applied to clinical practice to improve patient outcomes.
Recap: Updates in Treatment of HER2-Positive Breast Cancer and Brain Metastases
July 16th 2022Sara A. Hurvitz, MD; Stefania Maraka, MD; and Ruta Rao, MD, discuss the evolving landscape of metastatic HER2+ breast cancer, highlighting recent clinical trials and the management of patients with brain metastases.
Recap: Emory Experts Review Treatment Strategies for Transplant-Ineligible Multiple Myeloma
June 20th 2022A panel of experts from Emory University review several key data updates in multiple myeloma from recent meetings and discuss how the data can be applied to clinical practice to improve patient outcomes.